Introduction
The ovary is an organ with a functional capacity limited in time, normally ending around the age of 50, when most women experience natural menopause. The reason for this phenomenon is the depletion of the cellular structures that guarantee the two main functions of the ovary, reproductive and hormonal. These structures, called follicles, are in a basic and immature state since the constitution of the ovary during fetal life. Intermittently, some of these follicles begin a growth process that leads them to increase their volume, thanks to the multiplication and increase in size of their cellular components.
The most palpable changes occur in the somatic cells, which compose the granulosa and the theca layers, and that are responsible of the synthesis of hormones. This dynamic follicular expenditure begins before birth, and continues throughout life, also in childhood, until its exhaustion with menopause. It is not absolutely ruled out that there is a certain regenerative capacity of follicles, described in rodents [1,2], which has been confirmed more recently from pluripotent cells [3]; however, even if it exists in humans, it is obvious that its effect is very scarce. The other cellular component is made up of a single cell, the oocyte or ovum, which does not multiply, but undergoes an increase in size and a maturation process throughout follicular growth.
Follicular activation determines a series of waves of follicle growth, the so-called follicular cohorts, which progressively reduce the number of participants throughout the whole process, so that when the time of ovulation arrives, only one remains. The continuous follicle expenditure determines that the number of resident follicles in the ovary decreases with age. Changes in the cycle may occur when the hormone production of the follicular cohorts is deficient. If the process is shortened and ovarian failure is definitive before the age of 40, we speak of premature ovarian insufficiency (POI) [4].
Effect of ovarian aging
Ovarian aging causes dysfunctions that arise in the final phases of the operational life of the ovary. In women of the general population who experience natural menopause around the age of 50, the impact of ovarian aging becomes overtly apparent between 35-38 years of age. There are histological changes at the global level of the gland, such as a progressive increase in fibrosis and expansion of the stromal component, arteriolar obliteration, and changes in the medullary and cortex volume [5]. When there is still an apparently sufficient number of follicles in the ovary, at a functional level there is a relatively early deterioration. The decline in the number of follicles asymmetrically affects the two basic functions of the ovary, reproductive and hormonal. Hence, at a clinical level, there is a more rapid deterioration, around 10 years earlier, manifested in fertility. This temporal difference has been observed in population studies in which the median age of the last birth is close to 40, while that of menopause is close to 50 [6].
Impaired fertility
It is remarkable that fertility deteriorates despite the fact that there are no apparent differences between the follicles involved in late cycles (women 35 or more) and those activated 10 or 15 years earlier. There are post-ovulatory deficiencies that contribute to the deterioration of reproductive success (i.e. the clear increase in the rate of reproductive losses with age); but there are also previous dysfunctions, expressed as the difficulty in becoming pregnant. Therefore, there is less reproductive success, which is manifested in all steps of the process, that is, in the phases that determine the outcome of fertilization, and also in the post-fertilization phases, from implantation to the risk of abortion.
The reasons that determine the reduction of reproductive success are not clear, but, despite the limited knowledge of the involved details, the mechanisms that initiate the cohort activation, and the consequences of ovarian storage for long periods, are potential candidates.
Changes in hormone production
The second aspect is related to hormonal dysfunctions, which at a more advanced age can lead to changes in the endocrine production by the follicle. This includes estrogens and other hormonal species, such as inhibins A and B, produced by the granulosa and, when anovulatory cycle patterns are established, also progesterone.
It is assumed that the number of follicles that make up each growing cohort is proportional to the number of those that are still residing in the ovary. Therefore, as years go by, the number of follicles of each cohort decreases. This progressive contraction is reflected in the decrease of circulating levels of anti-Müllerian hormone (AMH), which parallels with the increase in age. This hormone is produced by growing follicles, and its expression is significantly reduced, and eventually abolished, by the growth of small antral follicles. AMH levels act as an indicator of the age-related reduction of the follicular cohort size. Whether age imposes a reduction in the follicular performance with an effect on the production of AMH is a hypothesis that cannot be excluded, although still unsubstantiated.
Although the cohort reduces in size, estrogen production usually changes little if the cycle remains ovulatory, since most of the estrogen output comes from the dominant follicle, which is present before ovulation. In fact, in an apparent paradox, there are even hyper-estrogenic states in the cycles near the end of female reproductive stage, as a consequence of the overproduction of follicle-stimulating hormone (FSH), which increases significantly in the few years prior to menopause. When the cohort lacks the capacity to guarantee ovulation, and does not generate sufficient estrogen levels, amenorrhea is established. Its duration may vary until another follicular cohort is capable of providing the estrogenic substrate to rescue bleeding, whether or not accompanied by ovulation. When rescue cycles do not occur within a period of one year, we speak of menopause. POI is considered if the loss of ovarian activity occurs in women under the age of 40. The persistent hypoestrogenic state will maintain or exacerbate the high levels of FSH, which at this stage are already accompanied by high levels of luteinizing hormone (LH).
Operational determinants in POI
The POI state is established as a consequence of the early depletion of the ovarian follicular mass. At the same time, the systems involved in the initiation and progression of follicular cohorts may present sufficient deficiencies to fail in their purpose, such that despite having an ovary with a normal follicular supply, it is incapable of generating successive follicle cohorts that mature until ovulation is achieved. Therefore, early depletion or dysfunction of the responsible mechanisms can lead to POI.
The hypotheses on the mechanisms under early follicular depletion or activation dysfunction mainly come from research in mammals, mainly rodents or primates. The case of primates is interesting, since they share with our species various aspects related to ovarian aging. Shared traits include the endocrine substrate of the menopausal transition and reduced fertility with age, but also increases in cycle irregularity, increases in weight and percentage of body fat content (with tendencies toward insulin resistance and glucose intolerance), alterations in the lipid profile, with increased low-density lipoprotein cholesterol (LDL-C) and decreased high-density lipoprotein cholesterol (HDL-C) levels, decreased dehydroepiandrosterone, presence of vasomotor symptoms, or protective responses to estrogen replacement after oophorectomy in terms of bone metabolism, lipid profile and cognitive changes [7].
Since the endocrinology of ovarian aging presents abundant knowledge gaps, grouping into one option or another, depletion or dysfunction, is not always possible. Likewise, in some cases both mechanisms are operational, making them mixed processes.
Determinants preferably linked to early follicular depletion
The launch of a follicular cohort requires a system of signals to specific follicles so that they initiate growth, while all others remain quiescent. Details of this process require sufficient knowledge of the dialogue between the oocyte and adjacent somatic cells, mainly, and very possibly due to their proximity, with the crown of primitive granulosa cells that surrounds the oocyte. The paracrine and autocrine interactivity is in all possibly very intense.
Experiences in mice have confirmed that AMH is a key factor in limiting the number of follicles that are activated in each cohort. In mice engineered to lack AMH (knock out of the AMH gene), the number of follicular cohorts initiating growth is accelerated, leading to POI and early ovarian failure [8]. Therefore, the reduction in the availability of follicles may be due to an initial reduction, for reasons that are not yet well known, or to an accelerated expenditure, as demonstrated by the mentioned experiences where AMH is absent. It is possible that accelerated expenditure is a preferential mechanism that completes depletion, consequently incurring in a partial or complete disappearance of the gonad, which can be seen in monosomy X0 patients (Turner syndrome and variants). The lack of a complete chromosome means the absence of a series of genes that are involved in the mechanisms of rest or initiation of follicular growth, which will determine early follicular shortage, even before birth. The ovary itself will not experience normal development, with a ribbon structure. The absence of primordial follicles, accelerated expenditure, or both, means that, when the vital moment arrives in which puberty must occur, there are no longer available follicles and therefore there is primary amenorrhea of ovarian cause. In some cases, the profile is not absolute, and there are more or less numerous groups of cells with a double pair of chromosomes. Depending on the relative abundance of these chromosomally normal cells, and in the case of the variant in which they are XX, there may be cycles for an undefined length of time, which do not usually reach 40 years, so they meet POI conditions.
Determinants preferably linked to deterioration in the follicular response (dysfunction)
Autoimmune disorders stand out among the specific dysfunctions that have the capacity to alter the response to FSH and other stimulant agents. They constitute a matter of vast research, although their prevalence, around 4%-5% of POI cases, is less than initially proposed [9]. They are usually associated, at least initially, with an acceptable ovarian reserve and low FSH. Functional abolition of the ovary results from the appearance of autoantibodies against cells with hormonal responsibility, or against enzymes involved in steroidogenesis, such as 21-hydroxylase, 17-alpha-hydroxylase and others [10]. These women have fluctuating hormone levels during initial phases, with higher levels of AMH than other forms of POI.
There are essentially two clinical forms. One of them includes other glands, and therefore are categorized as poly-endocrinopathies, with concomitant failure in the pancreas (type 1 diabetes), the adrenal (Addison’s disease), and/or the thyroid. The other is POI due to specific autoimmune disease of the ovary. It can occur in isolation, or associated with other immune diseases but not endocrine pathologies. The mechanisms of low, or no response to the selection stimuli of the follicles in the cohort and their subsequent growth are unknown [9].
Finally, there is a group of cases in which an endocrine pattern of hypogonadism may appear, therefore with elevated FSH, but apparently normal follicular supply. These cases present a mutation that inactivates the FSH receptor (FSHR) gene, which prevents a response to FSH despite the ovaries having normal follicular supply [11]. There are other cases, such as defects in steroidogenesis enzymes, which will not be described here.
Mixed determinants
The poor knowledge of most of the mechanisms operating in POI make that several of the working factors use pathways of which details are totally or partially unknown. This situation makes it difficult to assign them to the group of determinants that act due to depletion or dysfunction. Most likely, in several cases the determinants are mixed.
Mixed or not well-typed mechanisms include the premutation of the FMR1 gene, fragile X messenger ribonucleoprotein 1, which is associated with fragile X syndrome. These cases present a higher risk of POI, around 20% in carriers of the premutation [12]. The molecular mechanisms are not clear, but experiences in mice show that both follicular depletion and impairment of follicle growth are present [13].
The number of genes involved in the development of POI is continually growing, as revealed in studies of complete exomes or genomes in all their variants. There are those that are involved in the genesis of the main actors, gonad, oocyte or follicle, as well as in actions that involve various functions, such as apoptosis, metabolism, cell cycle progression, meiosis, hormonal signaling, among others [14,15]. The set of actors involved include FSH itself and the complex of paracrine and intracrine mechanisms that condition the activation of the follicle. Among them are the Kit ligand, also called stem cell factor (SCF) [16]. There is a notable range of agents from granulosa and oocyte that have demonstrated the ability to stimulate the growth of pre-antral follicles, including the tyrosine kinase receptor, the serine kinase receptor, or the wingless receptor, while the via Hippo, limits follicular growth. All of them act at different stages in the folliculogenesis process, but at the moment it is impossible to attribute to them a role of accelerated depletion or dysfunction [16].
Taken together, advances in this field, absolutely closed to research until a few years ago, can achieve improvements in clinical challenges of such importance as the low response to FSH in the treatment of infertility or for an undetermined number of patients with POI.
Oxidative stress and ovarian aging
The recent possibility of analyzing the transcriptomic changes that occur in each type of ovarian cell has concluded that, both at the oocyte and granulosa level, the functional deterioration that appears with ovarian aging is associated with oxidative damage [17]. These data confirm the theory proposed by Tarín [18] and by others. According to this hypothesis, the accumulation of reactive oxygen species (ROS) occurs continuously in the mitochondria as a consequence of the leakage of high-energy electrons produced in the mitochondrial electronic transport chain. There is sufficient molecular evidence, the details that exceed the purpose of this article, to confirm that the overproduction of ROS would condition a series of effects such as the inactivation and/or mutation of mitochondrial DNA, as well as the accumulation of abnormal proteins and affectation of the lipid component of cell membranes. Contrary to what is assumed, the oocyte is not metabolically inactive while residing in the ovary before being engaged in a follicular wave, and would accumulate this damage. Consequently, oocytes of the final reproductive cycles, which would have been subjected to this process in the ovary for longer time, would be strong candidates for presenting functional anomalies. Therefore, the lower fertilization or higher abortion rates observed in the clinical scenario would have a coherent explanation [18].
Clinical translation of the process of progression towards ovarian failure
Follicular cohorts present certain numerical heterogeneity among them. When ovarian reserve is optimal, in younger women, the number of follicles recruited is usually sufficient to guarantee a normal ovulatory cycle. When the number of follicles is reduced below a certain threshold, with or without a still unclear contribution of potential functional deficiencies in the concerned follicles, the entire growth and maturation processes suffer, and the rate of anovulatory cycles increases. There is a progressively downward trend in the number of follicles in the cohort, in a sawtooth process, with certain differences between some cycles and others. Therefore, sometimes a normal cycle will emerge, while in other cases, the cycle is irregular, with possible amenorrhea intervals. This is described in all ovarian aging processes, either natural, at the normalized population age, or early, as in POI.
This explains the abnormality of the cycles prior to definitive amenorrhea. There may be an abnormal, long or short cycle length, or bleeding abnormalities; and this, in addition to the possible alternations between normal and abnormal cycles mentioned above. There may even be a return to normal cyclicity after a period of months of amenorrhea. In POI, these periods of amenorrhea can last more than a year, with returns of the cycle once it has been considered lost. These amenorrhea periods are accompanied by the standard hormonal pattern of ovarian failure, low estrogen, and elevated FSH.
Moreover, some cases of POI occur when processes arise that involve massive destruction of follicles, such as after chemotherapy or radiotherapy, or an autoimmune mechanism that is set in motion and ends up also producing a more or less rapid abrogation of follicular functionality.
Endocrine patterns in the senescent ovary with possible reproduction in POI
The POI state implies that before the age of 40, the ovary lacks sufficient substrate, in terms of follicular supply, to maintain menstrual cycles. This would be a premature version of the natural state of a 50-year-old woman. Although scarcely studied, it is therefore expected that the phenomena that occur in POI are similar to what occurs in the process of establishing natural menopause, although around 10 years in advance.
The Stages of Reproductive Aging Workshop (STRAW) initiative, revised in 2012 [19], was sought to reach a consensus on the different stages that occur throughout a woman's reproductive life. To do this, experts proposed a series of defined clinical changes and their corresponding hormonal background.
In the case of the last years of ovarian functionality, the clinical stages were defined based on evidence obtained from consistent epidemiological studies, specifically the Study of Women Across the Nation (SWAN) [20], the Melbourne Women's Midlife Health Project [21], the Seattle Midlife Women's Health Project [22] and the TREMIN Research Program on Women's Health, which is the oldest open study on menstrual patterns [23]. It is important to learn from them because they can show warning signs about the imminence of POI.
STRAW consensus stages
STRAW divided women’s reproductive life into 3 phases, the reproductive phase itself, the menopausal transition and the postmenopause (Figure 1). Each phase was subdivided into stages. In relation to the topic at hand, the final stage of reproductive life and the menopausal transition are of interest.
Figure 1. The STRAW scheme.
In the final stage of reproductive life, small changes in the menstrual pattern can be detected, which can present shorter cycles and lighter bleeding. Hormonally speaking, the reduction in the number of follicles in the cohort results in lower levels of AMH. Added to this is a reduction in inhibin B, which can be accompanied by slight increases in FSH, mainly at the beginning of the cycle.
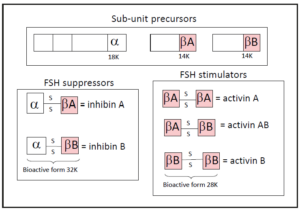
Inhibin B is a double-stranded peptide, whose subunits, α and β-B, are linked by a single di-sulfide bond. Each of the chains of inhibin comes from a larger pro-peptide, of which only a chain of 18 kDa (⍺ subunit) or 14 kDa (β subunit) will be available to join and generate the inhibin B. The β chain in turn has two isoforms, A and B, so that when the chain that joins with the ⍺ is A, inhibin A results, and when the one that joins is B, inhibin B results (Figure 2).
Figure 2. Molecular structure of inhibins A and B. When the dimer combination includes two β chains, it results in a peptide whose function is stimulating at various levels. This is activin, which can be A, B, or AB, depending on the combination, as shown in the figure.
Inhibin B specifically limits the synthesis and release of FSH. Its reduced production defines an endocrine fact, in principle characteristic of the end of ovarian life. It is not clear if it is a feature of the aging of the granulosa cells, only present around 8-10 years prior to the age of 50, or due to the state of follicular depletion prior to definitive ovarian failure. There is no clear data on this, but in the second case it would possibly be an operating mechanism in POI.
The increase in FSH acts by promoting the synthesis of estradiol in granulosa cells. This effect is at the basis of the increase in estradiol in sporadic cycles, which has been described by some authors in the menstrual cycles at the end of ovarian life [24]. Other factors operate in favor of this, such as the increase in aromatase activity [25], but there are other factors against it, such as the presumed decrease in the mass of granulosa cells as a consequence of the reduction in the number of follicles in the cohort, confirmed in part by the decrease in circulating AMH. However, the impact of this variable is uncertain, since most of the estradiol produced in each cycle comes from the step of follicular dominance, in which there is already a single follicle with evident operational potential.
Another aspect of interest is the reduction in the activity of the corpus luteum, which is attributed to a possible deterioration of follicular quality. It would be an additional actor that contributes to the increase in FSH. The reason is that the levels of progesterone in the final days of the corpus luteum, just prior to the next cycle, have a limiting effect on FSH. A defective corpus luteum is expected to produce less progesterone, resulting in increased FSH production [26].
Cycle alterations, when they appear, translate in different forms such as changes in the frequency of bleeding, in the magnitude of bleeding, or in both. Some women, about a third, describe no change at all. The trend, more or less pronounced and rapid, is towards an increase in anovulation rates and definitive amenorrhea [27]. Anovulation can occur in the context of acceptable estrogenic levels, in which case these cycles are accompanied by alterations in the bleeding pattern, or low estrogen levels, in which case they would manifest as amenorrhea.
This transition phase may already present symptoms of hypoestrogenism, such as hot flashes and, in hyper-estrogenic cycles, symptoms of breast tenderness and bloating.
Conclusion
The inability of the ovary to regenerate the follicular endowment imposes the phenomenon of menopause. In the case of POI, there is a series of factors that may act as conditions for a reduced follicular population. The final phases of the cycle can reproduce in POI phenomena similar to those of the menopausal transition observed in the general population, but this has not been specifically investigated. In some women, cycles do not even occur, so we speak of women with primary amenorrhea, where puberty never appears, since the follicular population is already minimal or non-existent at the age corresponding to that vital moment. Along with this quantitative factor, ovarian failure can also occur as a consequence of a dysfunction of the endocrine system, in which proper functioning requires the maintenance of the set of steps involved in the normal development of the follicular cohort. The molecular details are largely unknown, as are those of the components of the menstrual cycle [28].
One mechanism or another imply a complex system in which there are determinants of cellular aging along with other factors that are specific of ovarian tissue aging. There is much that is unknown, but also much progress has been made, making this a topic of very high interest for clinicians and reproductive biologists.