Introduction
More than 30 years from the first successful oocyte donation (OD) [1], the procedure has become part of the assisted reproductive technique (ART) toolbox, and the demand for oocyte donors is increasing [2]. According to data collected for the European IVF Monitoring Program for the European Society of Human Reproduction and Embryology, 11,475 OD cycles were performed in 2005 [3]; 22,323 in 2009 [4]; 56,516 in 2014 [5] and 80,641 in 2018 [6]. Meaningfully, Spain is the country contributing the highest number of oocyte donation cycles in Europe (37,618 in 2018) [6] and has an economic impact on IVF centers.
Oocyte donors are healthy women in the younger age without a diagnosis of infertility who will undergo ovarian stimulation to obtain oocytes. Considering donor’s claims [7], more comfortable protocols should be developed for controlled ovarian stimulation (COS) to improve the donor’s experience. These protocols should include fewer injections, lower dose of gonadotropins, the use of alternative routes of administration, and reduced number of visits to the clinics [7].
Currently, the protocol of choice for ovarian stimulation in OD is the use of exogenous gonadotropins and GnRH antagonists to inhibit endogenous LH secretion. The GnRH antagonist protocol has replaced the long GnRH-agonist protocol because it reduces the duration of treatment and the total dose of gonadotropins [8]. Furthermore, GnRH antagonist protocols enable triggering of endogenous LH peak for final oocyte maturation by means of a GnRH agonist bolus, avoiding the ovarian hyperstimulation syndrome (OHSS) [9].
A new approach in the last years has appeared to control the endogenous LH secretion. The so-called Progesterone Primed Ovarian Stimulation (PPOS), consisting in the administration of oral progestin [10] in cycles where there is no fresh embryo transfer planned. The efficacy of oral medroxyprogesterone acetate (MPA) 10 mg treatment to inhibit endogenous LH surge during gonadotropin stimulation was first reported in IVF patients in whom embryos were frozen for deferred embryo transfer [11]. PPOS protocols have been successfully applied with a wide range of progestins, such as MPA [11], micronized progesterone [12] and dydrogesterone [13]. PPOS have earned great interest in the context of OD because mature oocytes can be obtained with reduced number of injections and greater convenience [8,14–17], achieving 3 of 4 goals claimed by egg donors: including fewer injections by reducing the antagonist injections, the use of alternative routes of administration, changing de subcutaneous route by oral and reduced number of visits to the clinics, and the non-need to do an early appointment around day 6 to start the antagonist [7].
A recent metanalysis showed no differences in mean number of retrieved oocytes comparing PPOS with conventional GnRH antagonist protocol [8]. Oral administration of medication in the PPOS protocol is more attractive and cheaper than the daily subcutaneous injections of a GnRH antagonist [18]. Progestin use, may be cost-effective when freeze-only is planned such as in preimplantation genetic testing or fertility-preservation cycles where a GnRH antagonist protocol would otherwise be used [18]. In the OD program of our University Hospital, the use of oral desogestrel (DSG), a synthetic progestin, for PPOS was incorporated in 2017 [17].
Our objective was to conduct a cost-effectiveness study comparing progestin with conventional GnRH analogue egg donor IVF protocols, and if it is truly cost-effective owing to the higher gonadotropin use. Given the lack of published data critically assessing the cost-effectiveness of progestin protocols, the main outcome is the cost-effectiveness ratios as cost per mature oocyte and cost per treatment.
Methods
This was a retrospective cohort single center study carried out from 2012 to 2021 in a university affiliated private fertility clinic. The Institutional Review Board approved the study on 30 September 2021 (reference number CIOG FSD-IAO-2021-12). The present study analyzed the cost-effectiveness between GnRH antagonist ovarian stimulation protocol and PPOS protocol.
Eligible oocyte donors were aged 18 to and 35, with regular menstrual cycles, body mass index (BMI) between 18-30 Kg/m2, and no relevant medical history. The patients had a normal karyotype and fulfilled national legal requirements. Inclusion in the oocyte donor pool also required that the donor had at least ten antral follicles at the beginning of the cycle. Patients included in this study were oocyte donors who had undergone a stimulation cycle with recombinant FSH gonadotropins and antagonist or progesterone (desogestrel), identified from the electronic medical records database. Donors were instructed by nurse staff regarding the management of the drugs and the starting day of controlled ovarian stimulation.
The exclusion criteria were all those who did not fulfil the inclusion criteria for being egg donor in Spain and cycles where 2 different gonadotropins were used in the same cycle.
Donors were divided into 2 groups (antagonists and PPOS) and 4 subgroups according to the type of gonadotrophin and the type of used pituitary inhibition:
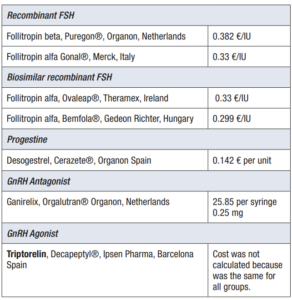
- Antagonist (Ganirelix, Orgalutran® Organon, Netherlands) Recombinant FSH (Follitropin beta, Puregon® Organon, Netherlands; Follitropin alfa Gonal® Merck, Italy) group
- Antagonist (Ganirelix, Orgalutran® Organon, Netherlands) Biosimilar recombinant FSH (Follitropin alfa, Ovaleap® Theramex, Ireland; Follitropin alfa, Bemfola® Gedeon Richter, Hungary) group
- Progesterone (Desogestrel, Cerazete®, Organon Spain) primed Recombinant FSH (Follitropin beta, Puregon® Organon, Netherlands; Follitropin alfa Gonal® Merck, Italy) group.
- Progesterone (Desogestrel, Cerazete®, Organon Spain) primed Biosimilar recombinant FSH (Follitropin alfa, Ovaleap® Theramex, Ireland; Follitropin alfa, Bemfola® Gedeon Richter, Hungary) group.
FSH stimulation treatment starts at the beginning of the follicular phase. Starting dose of recombinant FSH (r-FSH) was between 100-225 IU. Ultrasound scans and serum estradiol determinations in specific situations were performed for response monitoring from stimulation day 6 and repeated as needed until meeting the criteria of final oocyte maturation triggering (i.e. >3 follicles of > 18 mm). Pituitary suppression was performed under an antagonist protocol or desogestrel. Trigger was performed with a GnRH-agonist bolus (triptorelin 0.2 mg; Decapeptyl®, Ipsen Pharma, Barcelona Spain) and oocyte retrieval was performed 36 hours later. The retrieved oocytes were fertilized with the partner's sperm or with donor sperm depending on the patient's medical history, and fresh or delayed transfer was performed in the recipient.
During the study period, a total of 5,678 OD cycles were performed at our institution, and only 3.6% were cancelled due to poor ovarian response (≤ 4 MII) and no oocytes were obtained in 1.17% of the cycles.
Demographic data as age, BMI, retrieved oocytes, metaphase II oocytes retrieved, total dose of r-FSH, days and types of medication were analyzed for each group.
Statistical analysis
All statistical tests were applied with a confidence level of 95% and IBM SPSS version 26.0 statistical software (IBM Corporation, New York, USA, 2023) was used for all analyses. All variables were analyzed and a Spearman's Rho correlation was used. In the non-parametric analysis of the four groups, the Kruskal Wallis test was used. The Mann-Whitney U test was used to compare the total cost of medications.
Economic evaluation
The cost per unit of gonadotrophin was calculated according to the manufacturer’s cheapest sale price per unit at the date of 12/2019. The costs for each drug are given in Table 1. (Table 1)
Results
Demographics of the oocyte donors
A total of 5,678 women, with a mean age of 26.5 years, were recruited into the study. The distribution into the different study groups was as follows:
- Antagonist recombinant FSF (r-FSH) group (n=3,155)
- Antagonist biosimilar recombinant FSH group (n=1,489)
- Progesterone primed recombinant FSH group (n=187)
- Progesterone primed biosimilar recombinant FSH group (n=846).
Of these study participants, 81.9% of donors were in the antagonist group and 18.1% of donors were in the primed progesterone group.
Comparative analysis of stimulation protocols in oocyte donors: clinical outcomes and medication usage
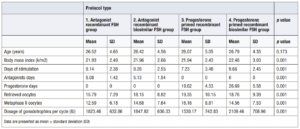
According to clinical outcomes in oocyte donor, we did not find statistical differences in the age and days of stimulation in each treatment group.
The number of retrieved oocytes, and Metaphase II oocytes were higher in the progesterone primed FSH group (18.87 ± 9.53; MII 14.85 ± 7.76) than in the antagonist group (16.54 ± 7.78; MII 13.26 ± 6.65) without statistical significance, but a higher dose of gonadotrophins was required (2,005.19 ± 715.1 IU vs 1,831.25 ± 633.97 IU). After analyzing all variables, a positive correlation of 0.728 according to Spearman's Rho was found between days of stimulation and dose. The rate of mature was 0.8 in the antagonist group compared to 0.78 in the PPOS group. Results of clinical outcomes of groups are shown in Table 2. (Table 2)
In the detailed analysis of the subgroups, a deviation is observed in subgroup 3, with fewer days of stimulation and more mature oocytes obtained. In the non-parametric analysis of the four groups, the Kruskal Wallis test was used. A significance in favour of group 3 was seen in BMI, days of stimulation, retrieved oocytes and metaphase II oocytes (Table 3).
Between subgroup 1 and 2 the stimulation days and antagonist days were similar with similar use of gonadotrophins.
Of note, subgroup 4 required the highest dose of gonadotrophins per cycle (2,109.46 ± 708.96 IU).
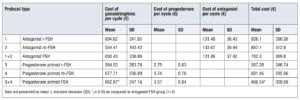
Table 4 shows the used medication according to each protocol subtype. (Table 4)
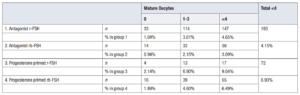
Of these study participants, 193 donors (4.15%) antagonist FSH group and 72 donors (6.93%) in the progesterone primed FSH group had a sub-optimal result (less than ≤ 4 MII collected) that did not enable the oocytes to be allocated. The percentage of cycles in which no mature oocytes were obtained was almost twofold in the progesterone group (1.01% vs 1.9%) (Table 5).
Economic evaluation
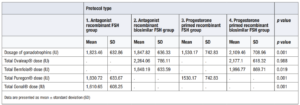
The gonadotrophin cost in the antagonist FSH group was 650.43 ± 236.86 € and in the progesterone FSH group it was 662.97 ±247.16 €. Statistical significance p=0.037 was observed between the two groups and was also observed when calculating the total cost of medication using de Mann-Whitney U analysis (p=0.001). When comparing the subgroups, there were no differences between groups 1 and 2 in the number of days of antagonist use or the administered doses; hence, this had no impact on the cost difference. Despite this, when comparing the number of days of progesterone use between groups 3 and 4, group 4 used more days, which resulted in a higher cost. Nevertheless, the increased cost was not significant due to the low cost of desogestrel. There were no significant differences in total medication costs between groups 2 and 3 (p=0.114), but there were significant differences between the other subgroups (p=0.001) (Table 6).
At first, we considered a suboptimal result when the oocyte pick-up was fewer than five oocytes. In the progesterone FSH group oocyte pick-up was 2.78% higher than the antagonist FSH group, meaning an over increasing cost of 13.7 € per cycle in the progesterone group. The average cost charged to the total cycle from suboptimal cycles with progesterone was 46.2 €, and in case of suboptimal cycles with antagonists it was 32.5 €.
The cancelation rate in a started cycle was 4.15% in the antagonist FSH group and 6.93% in the progesterone primed FSH group. Giles et al. [16] reported 3.2% and 2.4% respectively and Begueria et al. [15] reported just 6.6% in the antagonist group but the n was low.
The cancellation of the cycle has a direct impact on the total cost of each group because an expense has been incurred without being able to assign oocytes. This expense is spread over the rest of the cycles in which oocytes are obtained.
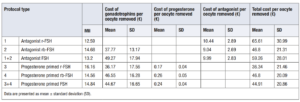
Second, we considered the price per retrieved oocyte is provided in order to refine the analysis and give a real value of how much it costs to obtain an oocyte with each protocol. The cost in the progesterone primed FSH group was 14.35 € lower than the cost in the antagonist FSH group. The cost of the antagonist was 41 times more expensive than progesterone (Table 7).
In summary, results showed that the economic cost per treatment (Table 6), and the cost per metaphase II collected oocytes (Table 7) was significantly higher with the antagonist FSH protocol than with a progesterone primed protocol.
Discussion
There is an increasing demand for donor oocytes due to the widespread delay in childbearing in developed countries and new family structures [6], the difficulty for patients to access assisted reproduction treatments, a changing world with rising inflation and the difficulties of undersupply in some countries combined with problems of availability of some drugs. These situations require to bear in mind an economic perspective. The aim is not just to find the cheapest treatment, it is to find the treatment that has the same outcomes at the lowest cost. There is a growing acceptance that health policies and planning should take economic considerations into account.
This study found that progesterone primed protocol is more cost-effective than antagonist protocol. The assumption of cost effectiveness of PPOS over conventional ovarian stimulation cycles is based on the lower cost of progestins when compared with GnRH analogues, especially GnRH antagonists [19]. PPOS was only more cost effective than the GnRH antagonist protocol in all planned freeze cycles and egg donation. The cost-effectiveness analyses assumed similar live birth rates with PPOS, the short GnRH agonist and GnRH antagonist protocols, similar gonadotrophin consumption with PPOS and GnRH antagonist protocol [19].
To our knowledge, the present study is the first to be performed in relation to donors analyzing just cost-effectiveness between PPOS and antagonist protocol. Reviewing published comparative studies between both protocols in oocyte donors we found several studies without differences in days of stimulation, doses of used gonadotrophins and mean number of mature retrieved oocytes, except in the study of Yildiz et al. [14].
Begueria et al. [15] performed a randomized clinical trial (RCT). Treatment with MPA (10 mg/day) was compared with the antagonist protocol (Ganirelix, 0.25 mg/day, from day 7 of stimulation) for ovarian stimulation in OD. In total, 216 were randomized and 173 reached oocyte retrieval. The required days of treatment and total dose of r-FSH were 11.20 days on average in both groups. Treatment with MPA was comparable to ganirelix in terms of number of metaphase II (MII) obtained oocytes (15.1 ± 8.3 with MPA versus 14.6 ± 7.0) with ganirelix [15].
Yildiz et al. [14] performed a retrospective study comparing the use of MPA administered in the form of the flexible antagonist protocol for the treatment of oocyte donors and the clinical outcomes in recipients of fresh oocytes. Each donor was stimulated with the flexible GnRH antagonist protocol in one cycle and with the new flexible-PPOS (fPPOS) protocol in the other, within a period of 6 months. They received FSH 225 IU from cycle day 2–3, and 0.25 mg/day GnRH antagonist or 10 mg/day MPA started on stimulation day 7 or when the leading follicle reached 14 mm, whichever came first. There were no differences in the duration of stimulation and total gonadotrophin consumption, the PPOS was 2,475 IU (2,250–2,475 IU) and the antagonist group 2,400 (2,250–2,475 IU). The fPPOS yielded a significantly higher number of cumulus oocyte complexes than GnRH antagonist cycles (33 [range 21–39] vs 26 [18–36], respectively) [14].
More recently Giles et al. [16] performed one RCT including 318 oocyte donors and compared MPA 10 mg/day with the use of a GnRH antagonist and found no significant differences in FSH dose (1,964 ± 431 IU vs 1,973 ± 392 IU, respectively), neither in the days of stimulation (10.0 ± 1.5 vs 10.1 ± 1.3 days), nor number of retrieved oocytes (21.4 ± 11.7 vs 21.3 ± 9.3, respectively, p=0.949).
Finally, Castillo et al. [20] performed a retrospective analysis comparing the use of 200 mg oral micronized progesterone with cetrorelix in 1,090 oocyte donors, and found no differences in the number of oocytes (15.8 ± 7.5 versus 15.2 ± 7.6, respectively, p=0.70).
A meta-analysis of the previous studies comparing PPOS with GnRH antagonist protocols for the treatment of 2,147 oocyte donors and 2,260 recipients was performed and showed no differences in mean number of retrieved oocytes (mean difference 0.23, [95% CI 0.58, 1.05]) [8].
The results of our research are shown to be in line with those of the authors with the same donor population in days of stimulation 9.2 ± 2.4 vs 9.2 ± 2.6 days, and mean number of mature retrieved oocytes 13.2 ± 6.6 vs 14.8 ±7.8, corresponding to the antagonist group FSH vs progesterone primed FSH, respectively, but less dose of gonadotrophins is used in antagonist group 1,831 ± 663 vs 2,005 ± 715 IU. This might be explained by the fact that in some cases progesterone is started days before the start of ovarian stimulation and the ovaries may be slowed down.
According to the objective of our analysis, the economic assessment in the FSH antagonist group, showed that the cost of gonadotrophins per mature retrieved oocyte was 49.27 ± 17.94 € and the cost of medication per mature oocyte retrieved was 59.26 ± 28.01 €. In the FSH primed progesterone group the cost of gonadotrophins per mature retrieved oocyte was 44.67 ± 16.65 € and the cost of medication per mature retrieved oocyte was 44.91 ± 20.86 €.
If we perform an extrapolation exercise of the 80,641 oocyte donation cycles performed in Europe in 2018 [6], assuming that they were performed with antagonists and extrapolating our data with an average of 14 MII oocytes per cycle and a difference in cost per oocyte of 14.35 € between the two protocols, we would be saving 16’200,776.9 € annually without affecting the outcome.
The cost-effectiveness result of our study is in line with other published studies in egg donors [21] and fertility preservation with a saving in the cost of stimulation using PPOS [22].
The cost of monitoring that included the costs of nursing, infrastructure, patient care time and consumables were not taken into account in our study and were considered comparable between groups. Both groups required the equivalent days of stimulation. One must bear in mind that in our center follicular monitoring starts on day 6 of stimulation for all groups, although this calendar at the beginning was determined by the fact that stimulation was performed with flexible antagonist introduction. Various published studies also support the flexible introduction of progesterone [14]. In our center, progesterone is started at the time of the menstruation or even 5 days earlier if the patient has been using hormonal contraception and has been switched to progesterone. This shows the flexibility in use.
Typically, a one-size-fits-all standardized approach is used to schedule multiple donor appointments during monitoring. Further studies are needed to optimize the number of monitoring controls to optimize the aggregate costs involved in the monitoring visit. Artificial Intelligence might offer the opportunity to influence workflow and scheduling during the stimulation process with clinical data, across operational and clinical boundaries. Outcomes could be optimized, efficiencies improved, and costs reduced through more tailored and patient-specific scheduling [23].
On the other hand, attention has increasingly been focusing on the improvement of the quality of care and the patient experience, in our case donors experience, including treatment.
Nowadays, the focus is on reducing injections, thus OS or the suppression of the LH peak, hence, LH surge suppression with oral progesterone administration instead of the subcutaneous route needed with antagonists has been reported to increase the level of donor satisfaction because of its simplicity [7]. Higher patient comfort and lower costs seem to be more attractive, moreover if there is need for less monitoring visits during treatment and better adherence to it in order to obtain similar outcomes compared to antagonist protocols. Even so, evidence on safety is essential [8].
Strength and limitations
We assessed the cost effectiveness of two different protocols, comparing PPOS and the antagonist protocol, however, data on this topic is quite limited. Despite this, our study has some limitations that are cause for caution. First, the retrospective nature of the design impedes the exclusion of selection bias; second, data are observational; and third only one medical center was involved, which may limit the possibility of generalizing our results. Therefore, further studies would be needed to establish causality or to determine the optimal protocol in order to achieve the best outcomes. Despite this, in our study progestins presented as an effective option for egg donation programs in terms of cost and the sample size is enough to validate our results.
It is important to note that the costs presented here are only a part of the total costs of the treatment, and other factors such as the cost of monitoring, anesthesia, and laboratory procedures also contribute to the overall cost. Plasma E2, LH or P4 data have not been included in the study because of missing data.
In conclusion, the study demonstrates that the PPOS protocol is more cost-effective than the antagonist protocol in oocyte donor programs. This finding is based on the lower cost of progestins compared to GnRH antagonists, while maintaining similar clinical outcomes. The study highlights the importance of economic considerations in selecting ovarian stimulation protocols, particularly in light of the growing demand for egg donation and the rising costs of fertility treatments. As such, the PPOS protocol presents a viable and more affordable option for clinics, potentially leading to significant cost savings without compromising effectiveness. Further research is needed to validate these findings and explore the broader implications for optimizing treatment protocols in assisted reproduction.