Introduction
First described in 1892 by Breus, massive subchorionic thrombohematoma, commonly known as Breus’ mole, has an incidence of 0.03-0.08%. It is characterized by a significant clot of maternal blood, measuring at least 1 cm, that separates the chorionic plate from the villous chorion [1-7]. Reports suggest that women with heart disease, circulatory disorders, hypertension, and diabetes may be more susceptible to develop Breus’ mole [4,7]. Additionally, there are case reports of recurrence in the same individuals, raising the possibility of a genetic or familial predisposition [7]. Nevertheless, the exact etiology and pathogenesis of Breus’ mole remain poorly understood [1,2,5,8].
Outcomes associated with Breus’ mole can include restricted fetal growth, oligohydramnios, preeclampsia, and intrauterine fetal death, that can vary depending on maternal age, gestational age, size of the hematoma, and, crucially, its location [1-5]. Diagnosis can be suggested through ultrasound; if imaging is inconclusive, magnetic resonance imaging may help differentiate it from other conditions [1,2,6].
Currently, there is no universally accepted definition for massive subchorionic thrombohematoma, nor are there specific criteria for its identification, as it shares characteristics similar to that of other pathological conditions [5]. However, when placentomegaly occurs alongside restricted intrauterine growth, Breus’ mole should be strongly considered in the differential diagnosis [2].
Given its association with miscarriage, preterm birth, and even chorioamnionitis, management strategies for Breus’ mole often include tocolysis, antenatal corticosteroids, and antibiotic therapy [3,4].
This document presents two cases of Breus’ mole and highlights the critical importance of diagnostic suspicion for effective management, despite its rare incidence.
Methods
Reports produced through a retrospective analysis of medical records. Both patients did not oppose the reporting of their cases, as it would not be possible to identify them.
Case report one
First case was a 32-year-old woman with blood type AB+, G2C1, gestational age (GA) of 24 weeks and 4 days, who was admitted to obstetrical emergency room, having been referred by the Fetal Medicine team due to uncontrolled maternal chronic hypertension, anhydramnios, early and severe fetal growth restriction, all leading to suspect the diagnosis of a Breus’ mole. She had been taking alpha methyldopa 2 g daily. Standard laboratory tests used to screen for preeclampsia revealed urine protein/creatinine ratio of 0.3 and uric acid of 6.1 mg/dL, with all other results within reference values.
An obstetric Doppler ultrasound performed on the day of hospitalization by the Fetal Medicine department revealed that fetal heartbeat (FHB) was present, the estimated fetal weight (EFW) was 310 g (p0), including the presence of anhydramnios, and a heterogeneous thickened placenta (6.2 cm), that was obstructing the internal cervical orifice. The Doppler scan of uterine arteries and the cerebroplacental ratio were pathological, without any description of ductus venosus but with increased peak systolic velocity (the value was not mentioned in the exam report). A medical report demonstrated placentomegaly, early and severe fetal growth restriction, findings of oligohydramnios/anhydramnios sequence (dolichocephaly, hypoplastic thorax, signs of bilateral ventricular myo-hypertrophy), and severe anemia due to severe placental insufficiency.
Prior laboratorial investigation of this patient was negative for TORCHS (toxoplasmosis, rubella, cytomegalovirus, herpes and syphilis), with a fetal karyotype 46,XY.
During her hospitalization, it was necessary to adjust the antihypertensive drugs due to recurring headache and uncontrolled blood pressure (association of alpha methyldopa 2 g daily, amlodipine besilate 20 mg daily, and levomepromazine 5 drops daily). On her fifth day of hospitalization, GA was 25 weeks +1 day. An ultrasound exam report pointed to anhydramnios, EFW of 412 g (p0), and a pathological Doppler velocimetry of the umbilical and middle cerebral arteries and abnormal cerebroplacental ratio, with no description of uterine arteries and ductus venosus. A choice was then made to administer a cycle of corticosteroid. At this point, the prognosis of an extremely premature birth was reiterated to the patient. Forty-eight hours later, another ultrasound was performed that indicated reversed-end diastolic flow and pathological ductus venosus (pulsatility index of 1.832), which led to the decision of terminating the pregnancy through a cesarean section.
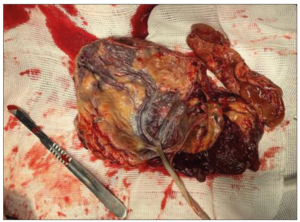
A cesarean section was performed without any incidents, with artificial delivery of the anterior previa placenta, that presented degenerated areas, a large subchorionic hematoma, and a velamentous cord insertion (Figure 1). The other surgical steps were uneventful. The baby was born alive, but evolved into death within a few minutes, due to extreme prematurity. The immediate postpartum period was uneventful and the patient was discharged, following the institution's protocol, in condition after 48 hours.
Case report two
The second case was a 29-year-old woman, G3P2, GA of 26 weeks and 5 days, also admitted at the obstetrical emergency room, having been referred by the Fetal Medicine with a suspected diagnosis of Breus’ mole due to an abnormal ductus venosus Doppler velocimetry, associated with restricted fetal growth and placentomegaly. She had a prior diagnosis of acquired thrombophilia and hypothyroidism, as well as a poor obstetric history due to anhydramnios and neonatal death, in her first pregnancy, and intrauterine fetal demise in her second one. She was on sodium Enoxaparin 80 mg day, acetylsalicylic acid 100 mg day, and sodium levothyroxine 100 mcg day,
Upon admission, laboratory exams demonstrated thrombocytopenia (93,000/mm3) and long partial thromboplastin time (48.90 seconds), with other routine exams for the screening of preeclampsia being normal, which dismissed a differential diagnosis of Mirror Syndrome, or Ballantyne. Laboratory investigation for TORCHS was negative. The antinuclear factor was positive (1:640, with a fine speckled pattern); the IgG anticardiolipin antibody was 280 GLP/mL and alpha fetoprotein was 352.1 ng/mL. No fetal karyotype was conducted.
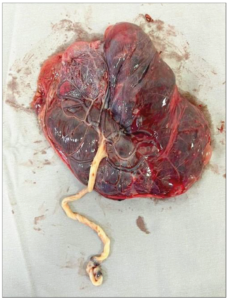
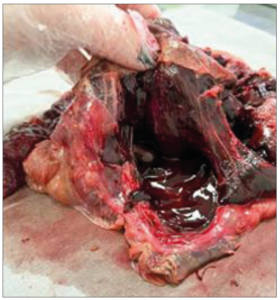
Doppler ultrasound on the day of hospitalization revealed the presence of FHB, an EFW of 700 g (p2), a maximal vertical pocket of amniotic fluid of 3.2 cm, a voluminous and thickened placenta (7.7 cm) taking up 40% of the uterine cavity, and a pathological Doppler, included ductus venosus. An ultrasound performed a week prior to the date of hospitalization revealed fetal growth restriction (p4), voluminous, thickened and heterogeneous placenta, in addition to an increased resistance of the uterine arteries and systolic peak of the medium cerebral artery. A cycle of corticosteroids was introduced due the possibility of early delivery. The choice was then made to deliver the infant through a cesarean section, which was performed without incidents. Following the placenta delivery, an important subchorionic hematoma was found as well as areas with degeneration and fibrosis (Figures 2 and 3). The infant born was alive, with a birth weight of 695 g, and an Apgar score of 7 at the first minute and 9 at the fifth minute, then referred to the neonatal intensive care unit.
During the immediate puerperium, it was necessary to introduce amlodipine besilate 20 mg daily. The patient was discharged 48 hours later in good condition, following the institution's protocol, and was instructed to keep antihypertensive, anticoagulant, and thyroid hormone medications.
Discussion
Although the pathogenesis of massive subchorionic thrombohematoma remains unclear, certain health conditions are significantly associated with its development, including heart disease, circulatory disorders, hypertension, and diabetes [1,2,4,5,7,8]. The prognosis is influenced by factors such as the maternal age, gestational age, size of the hematoma, and, particularly, its location [1,2,4,5].
Typically, the clot forms near the point of the umbilical cord insertion, which can lead to cord compression, obstructing the umbilical vein and consequently decreasing fetal perfusion due to placental insufficiency [1-4]. There is also discussion regarding the possibility of the clot jeopardizing the first wave of trophoblast invasion or, further, that antibodies such as anticardiolipin and antinuclear antibodies may increase platelet aggregation, resulting in thrombosis and/or vasculitis, which can lead to vascular rupture and the formation of a hematoma, albeit Breus’ mole has been described in patients with thrombophilia who are on anticoagulants [1,4,5,9,10]. Adds to that, another finding associated with the condition is the elevated serum level of alpha-fetoprotein [5,6].
Since case report one was our first, some tests were not requested, preventing us from comparing these two. In the first case, we only had information regarding chronic hypertension with superimposed preeclampsia, while in the second case, in addition to the knowledge of thrombophilia, we identified positivity for antinuclear factor, anticardiolipin antibody, and alpha-fetoprotein.
Potential outcomes include restricted fetal growth, oligohydramnios, preeclampsia, and intrauterine fetal death [1-5]. All these conditions are primarily linked to poor fetal prognosis, with Breus’ mole being an extremely rare cause of maternal life-threatening complications [7].
During ultrasound examination, Breus’ mole may appear as a heterogeneous, homogeneous, or hypoechoic intrachorionic mass, distinguishable from normal placental tissue, often accompanied by placentomegaly (thickness > 4 cm in the second trimester and > 6 cm in the third trimester), as seen in our two reported cases and others described in the literature [1,2]. If ultrasound findings are inconclusive, magnetic resonance imaging can be utilized, as it effectively differentiates Breus’ mole from other conditions, such as placental abruption, chronic abruption-oligohydramnios sequence, or placental mesenchymal dysplasia, making it the preferred diagnostic tool when available [1,2,6,9].
As noted, there is no standardized definition for massive subchorionic thrombohematoma (Breus’ mole) nor specific criteria for its identification, as many characteristics overlap with other pathological conditions [5,8]. For instance, placentomegaly can also occur in cases of hydrops, syphilis, toxoplasmosis, cytomegalovirus, parvovirus, and mesenchymal placental dysplasia; thus, these conditions must be ruled out, as we did. However, when placentomegaly is associated with restricted intrauterine growth, the differential diagnosis for Breus’ mole must be strongly considered [2,9].
With massive subchorionic thrombohematoma, approximately 60% of pregnancies result in survival after birth, although only about 20% reach full term [1]. When umbilical artery flow upon Doppler ultrasound is normal, and there is no fetal growth restriction, cases are associated with a favorable perinatal prognosis [1,2]. A review of the literature indicates that the average gestational age at delivery for cases of Breus’ mole is 29.9 weeks, with an average birth weight of 1,076 g. Furthermore, restricted fetal growth occurs in 55.8% of cases, with a mortality rate of 46.5% [2].
Given its association with miscarriage, preterm birth, and even chorioamnionitis, management strategies for Breus’ mole often include tocolysis, antenatal corticosteroids, and antibiotic therapy [3,4]. In both cases, only antenatal corticosteroids were prescribed.
Conclusion
Breus’ mole is rare condition that is more likely to occur in pregnant women with high blood pressure and circulatory disorders, similar to our patients. The typical progression includes placentomegaly, preeclampsia, restricted fetal growth, and oligohydramnios. Ultrasound examinations indicated that the placentas were thick, and although there are no reported correlations, we observed extensive areas of placental degeneration in these cases. No magnetic resonance imaging was performed, which might have confirmed the suspected diagnosis.
Additionally, TORCH infections and chromosomal changes were investigated and ruled out as possible differential diagnoses. In the second case, increased levels of alpha-fetoprotein, antinuclear factor, and anticardiolipin antibodies were observed, although defined diagnostic criteria for Breus’ mole are still lacking.
In the first case, unlike most reports in the literature, the perinatal prognosis was not good, and the newborn died a few minutes after birth, possibly due to extreme prematurity, very low birth weight, and altered perinatal Doppler velocimetry. In the second case, however, the newborn had a better immediate outcome, with an expectation of improvement in the ICU, despite sharing many of the complicating factors mentioned above, although we are unaware of the final outcome.
As the pathogenesis of the condition is not yet fully understood, little can be done in terms of prophylaxis. However, we should indeed suspect the condition so that potential cases can be better managed. Therefore, to achieve different outcomes in the future, more in-depth studies need to be conducted on this matter, which may be challenging due to its rarity.