Introduction
Adenomyosis is a pathologic condition defined by the presence and growth of endometrial or endometrial-like structures within the myometrium [1]. The advent of imaging modalities, such as magnetic resonance imaging (MRI) and transvaginal ultrasound (TVUS), has led clinicians and researchers to realize that adenomyosis is not exclusively a pathology of multiparous and elderly women [2]. The prevalence of adenomyosis may vary from 5% and 70% (mean 20%–30%), basically due to the complexity of the diagnostic process [3]. Additionally, adenomyosis has been reported to have a prevalence of 2-4% in postmenopausal women [4].
Adenomyosis lesions originate from the basal layer of the endometrium that invaginates into the weakened junctional zone, as a result of tissue trauma or a predisposition of the myometrium, thus enabling endometrial tissue to migrate [1]. Upon diagnostic imaging, adenomyosis displays an enlarged uterine corpus with a globular configuration, with diffuse endometrial infiltrates which may predominate in one uterine wall or as focal nodules. Due to muscular hyperplasia and hypertrophy in adenomyosis patients, the myometrial vascularity is generally increased [3].
Estrogen is essential for the establishment and growth of the ectopic endometrium outside the uterine cavity. Increased estrogen receptors (ERs) and/or a decrease of progesterone receptors (PRs), along with modulation of the immune system, likely contribute to the development and growth of adenomyosis lesions [5,6]. Tamoxifen, a selective estrogen receptor modulator (SERM), acting as an agonist to ERs in the uterus, induces adenomyosis in mice [7] and in a group of postmenopausal patients with BC who underwent total abdominal hysterectomy and bilateral salpingo-oophorectomy after tamoxifen treatment a higher incidence of adenomyosis was observed compared to controls [8].
Tamoxifen is used as an adjuvant endocrine therapy for BC patients, effective in reducing recurrence and increasing survival of ER-positive BC patients. Long term tamoxifen therapy (5-10 years) is associated with low recurrence and mortality rate [9]. Due to the hormonal stimulation of endometrial tissue, individuals undergoing tamoxifen treatment for BC may face an increased risk of developing endometrial pathologies, such as endometrial polyps or endometrial cancer; thus, clinical and imaging surveillance is highly suggested [8,9]. Due to the possible contribution of adenomyosis on the development of endometrial polyps or endometrial cancer [10], a more close and careful monitoring of BC patients on tamoxifen are needed. Adenomyosis is detected in postmenopausal women by TVUS and MRI, showing similar molecular characteristics compared to reproductive age women [11].
The present study investigated the effect of long treatment with tamoxifen in BC patients on the appearance of newly developed adenomyosis diagnosed by imaging methods.
Methods
Subjects
The study group consisted of BC patients (n=25) aged between 40 and 76 years (mean 51.9 ± 8.11 years). The inclusion criteria were: treatment with tamoxifen for a period of at least 6 months and TVUS for the evaluation of endometrial thickness with abnormal uterine imaging findings, further assessed with MRI. Anamnestic data (body mass index [BMI] 27. ± 6.6 kg/m2), the presence of comorbidities and imaging findings were collected. All patients included in study had a TVUS before starting the tamoxifen treatment demonstrating no signs of adenomyosis.
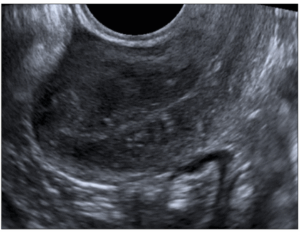
Pelvic assessment was performed by 2D/3D TVUS (Toshiba Aplio 400), using the standardized Morphological Uterus Sonographic Assessment (MUSA) group terminology [3,13] to describe the sonographic features of adenomyosis. The diagnosis was based on the presence of direct signs: myometrial cysts, hyperechogenic islands, or subendometrial lines or buds. Additionally, indirect indicators were also assessed such as globular uterus, asymmetrical myometrial thickening, fan-shaped shadowing, translesional vascularity, irregular junctional zone (JZ), or interrupted JZ at 3D ultrasound [3,12].
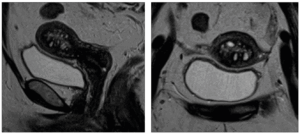
The MRI (Siemens Aera 1.5T) evaluation was performed in the group of 25 patients, according to the European Society of Urogenital Radiology ESUR guidelines [13] and included: turbo spin-echo (TSE) T2-weighted sequences on sagittal, coronal and axial planes, and T1 and T1 FS (fat-saturated)-weighted sequences. Contrast-enhanced T1 FS sequences (VIBE) were used in case of T2-weighted atypical features or in case of contemporary presence of other pelvic lesions, such as polyps or ovarian cysts. Diffusion-weighted imaging (DWI), allowing the mapping of the diffusion process of molecules, was used to differentiate adenomyosis from endometrial and myometrial cancer. Other direct and indirect signs typical of adenomyosis by MRI were also considered. These other considered features, which often overlap with those viewable with TVUS, were endometrial rim thickness, asymmetrical myometrial thickening, globular uterus, and diffuse or focal adenomyosis [13,14].
Results
During the course of follow up among the group of selected patients that used tamoxifen the TVUS examination evidenced the appearance of signs of adenomyosis: diffuse adenomyosis in 10 (40%); cystic adenomyosis in 17 (68%); adenomyoma in 7 (28%); thickened JZ in 5 (20%); lines and buds in 16 (64%); fan-shaped shadowing in 2 (8%), and echogenic islands in 14 (56%) (Figure 1).
Adenomyosis was confirmed by means of MRI examination, with more details including: globular uterus in 1 (4%); asymmetric type in 2 (8%); thickened JZ in 21 (84%); adenomyosis in 24 (96%): diffuse adenomyosis in 10 (40%); cystic adenomyosis in 23 (92%); adenomyomas in 6 (25%) (Figure 2). Other associated pathologies included uterine fibroids, endometrial polyps and/or hyperplasia.
Discussion
The present study showed the appearance of signs of adenomyosis in BC patients treated with tamoxifen through imaging technics (TVUS and MRI). Endometrial changes may occur during tamoxifen treatment for BC patients [9,15]. TVUS and MRI showed the same percentage of diffuse adenomyosis (40%), but with more details observed upon MRI: more cases of cystic adenomyosis and thickened JZ.
Indeed, TVUS has a good sensitivity and specificity when evaluating adenomyosis, corresponding to 64%-72% and 81%-84% respectively, when at least one sonographic criterion and two studies when two sonographic criteria are present [16,17]. TVUS represents a minimally invasive, easily accessible, and inexpensive examination; however, the most important limitations are the operator-dependence in terms of the definition of adenomyosis type and JZ thickness, beyond the contemporary presence of other pathologies that make it difficult to well define these features.
The JZ is the same structure evaluated by TVUS, MRI and histology. The morphometric measurements of the low-signal-intensity band in MRI corresponds to the area composed of dense smooth-muscle fibers in the inner myometrium on histopathological slides [18,20].
Modern imaging techniques allow the non-invasive diagnosis of adenomyosis and MRI has a central role thanks to its high diagnostic accuracy in the detection of adenomyosis and uterine disorders. By MRI it is possible to identify the main direct (cystic component) and indirect (JZ features) signs of adenomyosis and discriminate the composition and location of various adenomyotic features [13].
Standardized MRI protocol for the evaluation of adenomyosis, beyond endometriosis, has been developed. This protocol allows a proper interpretation of MRI images, minimizing possible pitfalls, such as focal JZ thickening due to transient uterine contraction, or such as the “pseudoenlargement of the endometrium” sign, represented by hyperintense linear striations radiating from the endometrium to the myometrium [13].
MRI is considered the most reliable, with higher sensitivity (77%) and specificity (89%) and a lower operator dependence. MRI shows elevated soft-tissue differentiation, also allowing the detection of uterine fibroids or endometriosis, the differentiation between various subtypes of adenomyosis, and the evaluation of surrounding pelvic structures, beyond an improved identification of the JZ layer [6,14,20. Our study confirmed the higher ability of MRI than TVUS in evaluating JZ thickness, thanks to its high contrast resolution.
In conclusion, the present study suggests that in some BC patients undergoing long term tamoxifen treatment, imaging follow-up with TVUS showing abnormal uterine findings further assessed by MRI may be useful in identifying adenomyosis.