AEEM Executive Board
Pluvio Coronado, Silvia González, Sonia Sánchez, Esther de la Viuda, Ana Rosa
Jurado, Jesús Presa, Francisco Quereda.
ESG Board of Directors
Camil Castelo Branco, Nathalie Chabbert-Buffet, Andrea Genazzani, David Serfaty, Marc Brincat, Angelos Daniilidis, Blazej Meczekalski, Andrea Giannini, Stefano Angioni, Geraldine Brinchant, Žana Bumbulien, Frédéric Chantraine, Kristina
Gemzell-Danielsson, Alessandro Genazzani, Attila Jakab, Ludwig Kiesel, Raoul
Orvieto, Lali Pkhaladze, Petra Stute, Tetiana Tetarchuk, Ineta Vasaraudze, Yves
Ville, Svetlana Vujovic, and Tevfik Yoldemir.
Introduction
Premature Ovarian Insufficiency (POI) refers to a situation of menstrual alterations (amenorrhea or oligomenorrhea), hypoestrogenism and elevated gonadotrophins due to the decline of ovarian function before the age of 40 [1-3]. This term describes a spontaneous or induced condition of the loss of ovarian function. Since this condition is not necessarily permanent, the term “premature menopause” should be avoided, as it is possible to restore ovarian function (usually sporadically), even after long periods of their inactivity [4]. In addition, this term does not generate the negative psychological impact of other names used above, since the word “insufficiency” conveys the idea of a dysfunction that is not always definitive, therefore it is the most appropriate word when describing the variability, reversibility and ovarian nature of the syndrome.
POI is considered a rare condition, and consequently studies on its incidence are scarce. Its prevalence varies according to the age of women, and it is estimated that it may affect 1 in every 10,000 women under the age of 20, 1 in every 1,000 women between 20 and 30 years, and 1 or 2 every 100 women between 30 and 40 years of age [1,3]. According to the latest data, there is an increase in POI incidence among the general population, placing it at around 2-3.7% [4]. The increased survival in malignant diseases diagnosed and treated in childhood with gonadotoxic therapies contributes to this higher frequency; ethnic, environmental and genetic differences have also been observed, with POI being up to three times more frequent in monozygotic and dizygotic twins than in the general population (detailed and referenced in etiopathogenesis section).
Currently, there are some differences in the criteria used to define the POI. While there is agreement regarding the age (under 40 years) and the presence of oligomenorrhea or amenorrhea, there are disagreements regarding the values of FSH. Some scientific societies recommend considering an FSH value greater than 40 IU/L on 2 occasions separated by at least 1 month [1,2], while others recommend an FSH value greater than 25 IU/L on 2 occasions separated by at least 4 weeks [3].
The aim of the present position statement is to provide health professionals with an explanation of the state of art in the management of patients diagnosed with POI and to improve and standardize the treatment and follow-up procedures for this rare syndrome throughout Spain. This position statement can serve as a reference for patient associations, family doctors and specialists to develop specific patient-centered care protocols. However, this document cannot be considered as a unique reference in case of all specific etiologies, comorbidities and related diseases, treatment options, other societies’ protocols; moreover, this is the second statement promoted by the Spanish Menopause Society
(https://aeem.es/wp-content/uploads/2022/08/menoguiamenopausiaprecoz_compressed.pdf) and it will need further updating in the light of new data. This statement for POI has been designed following the Delphi methodology, published in its complete form by the Spanish Menopause Society in 2024, available at https://aeem.es/wp-content/uploads/2024/05/Menoguia-IOP-electronico.pdf It comprises all the literature data analyzed in drawing up the AEEM Statement.
Endocrinology of ovarian aging
The ovary is an organ with a double function: endocrine and reproductive. A key characteristic of the ovary structure is that follicles, containing oocytes and hormone producing cells, can develop only until mid-fetal life. Stromal cells gradually decrease the production of androgens until the end of life. The follicular reserve undergoes a progressive decline until midlife; with the follicle reserve lower than 1,000, which happens around the age of 50 when menopause occurs. The oocytes of follicles from cycles occurring in the last reproductive years suffer functional deterioration, resulting in reduced fertility potential [5]. The underlying mechanisms remain unclear, although oxidative stress (OS) appears to play a decisive role. Recent data have substantially clarified the molecular details of this effect [6]. The impact of OS on hormone-producing cells impairs their functional capacity a few years later, during the menopausal transition, with a significant reduction in hormone production at menopause. Genomic instability, telomere attrition, epigenetic and cell communication defects are also implicated in the ovarian aging. Clinically, the evolution of ovarian life has several features defined in the Stages of Reproductive Aging Workshop +10 (STRAW +10) initiative, which outlines the characteristics of menstrual patterns during each stage and references the corresponding fertility potential [7]. Early appearance of these dysfunctions is expected in cases of POI. Essentially, two types of deterioration processes are expected: ovarian depletion, characterized by an early reduction in follicle numbers, and ovarian dysfunction, where follicle numbers remain unaffected, but their functionality is impaired. In both cases, premature ovarian failure occurs before the age of 40 in cases of POI. The molecular mechanisms underlying depletion are unknown, but an accelerated expenditure of resident ovarian follicles appears to be linked to dysfunctions in Anti-Müllerian Hormone (AMH) [8]. Additionally, there is a long and growing list of local factors, whether autocrine or paracrine, that result in ovarian incapacity to maintain a normal follicular population. Similarly, follicular dysfunction has been associated with autoimmune processes that generate autoantibodies against hormone-producing cells or enzymes involved in steroidogenesis.
Etiopathogenesis
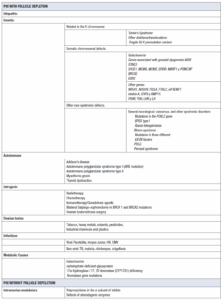
The etiopathogenesis of POI can be explained by a congenital decrease in the number of primordial follicles, impaired recruitment (lack of folliculogenesis), or accelerated follicular atresia (apoptosis leading to oocyte loss) [9]. POI may present with or without follicular depletion. In the first case, mechanisms can be triggered by genetic, autoimmune, iatrogenic, environmental, or toxic causes. Some genetic disorders are associated with abnormalities in the production of estradiol precursors or aromatase function, leading to decreased estradiol levels and the absence of physiological negative feedback on follicle stimulating hormone (FSH) (Table 1).
Genetic causes
Up to 30% of women with idiopathic POI have a family history of the condition, which implies a genetic etiology. This information is crucial as it may improve reproductive and genetic counseling. More than 50 genes associated with POI have been identified, mainly on the X chromosome and autosomal genes. Certain genetic mutations can affect gonadal development and function through various biological pathways. In patients with primary amenorrhea, the rate of abnormal karyotypes is higher than in those with secondary amenorrhea [10,11]. There is a strong relationship between the age of menopause, including POI, and permutations in the Fragile X mental retardation syndrome gene, specifically the messenger ribonucleoprotein 1 gene (FMR1) [11].
Autoimmune ovarian insufficiency
Autoimmunity was first indicated as a cause of POI when it was observed that some women with adrenal insufficiency (Addison’s disease) also had ovarian failure. The autoimmune disorder most associated with POI is thyroid dysfunction (14–27%), especially when adrenal autoimmunity is absent [12].
Iatrogenic
POI In recent years, the number of childhoods, adolescents and young adult cancer survivors has increased significantly. Many of them develop POI after oncologic treatment. In these cases, the pathogenesis of iatrogenic POI is primarily linked to an intense reduction in primordial follicles due to exposure to chemotherapy and/or radiotherapy. Another iatrogenic cause of POI is endometriosis, which can reduce the number of follicles either directly or through the effects of surgery. Thus, it is crucial to monitor ovarian reserve over an extended period following ovarian surgery [1]. Regarding post-surgical POI, prophylactic bilateral salpingo-oophorectomy is often performed in patients carrying BRCA1 and BRCA2 mutations. Although there is insufficient evidence, viral infections (i.e. mumps, herpes zoster, cytomegalovirus, chickenpox, HIV) or their treatments may cause oophoritis, and secondarily POI. POI has also been observed after non-viral infectious processes such as tuberculosis, malaria, and shigellosis [3]. There are case reports which associate viral infections such as mumps, with orchitis and testicular failure in men and which may suggest a link between these infections and oophoritis and ovarian failure in women. However, acute oophoritis is much more difficult to diagnose in women than orchitis in men, and most of the evidence that viral infections can cause primary hypogonadism in women is circumstantial [13].
Clinical presentation and natural history
POI is the premature cessation of ovarian hormonal activity in women under the age of 40, leading to a significant decrease in estrogen levels in the body. This early hypoestrogenism results in short-, medium- and long-term consequences, which will be discussed below. The earliest symptom reported by POI affected women is menstrual irregularities, ranging from irregular cycles to complete amenorrhea. Women with POI may also experience vasomotor symptoms, such as hot flashes. These symptoms originate in the hypothalamic thermoregulatory center due to decreased estrogen levels and increased luteinizing hormone (LH) hormone levels. A sudden sensation of heat in the face and neck occurs, associated with vasodilation and flushing, followed by excessive sweating and a subsequent drop in body temperature. Other symptoms related to the climacteric syndrome include night sweats, insomnia, mood disturbances, irritability, dermatological changes, vaginal dryness, sexual dysfunction, infertility, and psychological alterations [1,14]. Regarding psychological symptoms, it is essential to provide POI patients with proper counseling and treatment due to the significant impact on their mental health. Common psychological symptoms include mood swings, anxiety, depression, sleep disturbances, difficulty concentrating, and reduced self-esteem. Psychological and pharmacological treatment should be offered when necessary. In the realm of sexual health, estrogen deficiency may lead to the genitourinary syndrome of menopause (GSM). The sexual dysfunction experienced by these patients involves vaginal dryness, decreased sexual desire, reduced genital sensitivity, dyspareunia, dysuria, infections, and urinary tract disorders. As observed, all these symptoms are interconnected and often result from one another. Therefore, treatment should be holistic, addressing the condition comprehensively to improve the overall well-being of these patients [15].
Long-term consequences of POI
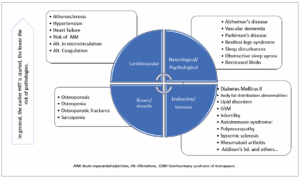
POI induces significant physiological changes that predispose affected individuals to earlier complications than women who experience menopause at a physiological age. These complications include decreased quality of life, cardiovascular events, neurodegenerative diseases, osteoporosis, sarcopenia, mood disorders, sexual dysfunction, and increased overall mortality (Figure 1) [1,2,16]. The emotional impact is crucial, making psychological support essential to improve adherence to hormone replacement therapy (HRT) and non-pharmacological treatments [17,18].
Cardiovascular risk
POI is an independent risk factor for cardiovascular events due to endothelial dysfunction, which may lead to atherosclerosis and hypertensive conditions. Women with POI have a 53% higher probability of experiencing coronary events compared to that present menopause at physiological age. HRT can reduce these risks if initiated before endothelial damage occurs. Studies suggest that cardiovascular risk increases by 2% for each year that menopause occurs earlier than age 50. Additionally, hypertension and metabolic syndrome are more prevalent in women with POI, leading to body composition changes and increased dyslipidemia [19-23].
Musculoskeletal risk
Estrogen deficiency negatively affects the musculoskeletal system, increasing the prevalence of osteoporosis, sarcopenia, and osteoarthritis. Muscle mass loss and bone mineral density (BMD) reduction are more pronounced in women with POI [24,25]. Compared to women who experience menopause at a physiological age, POI patients have a higher risk of osteoporotic fractures [26]. Lifestyle modifications and early initiation of HRT can reduce these risks, although their efficacy remains disputable [27].
Cognitive risks
Estrogens have neuroprotective effects, and their deficiency may contribute to cognitive disorders such as dementia and Alzheimer’s disease [28,29]. The use of HRT appears to reduce the incidence of mild cognitive impairment and Alzheimer’s disease. Additionally, POI may accelerate neurodegenerative diseases like Parkinson’s disease and increase the incidence of restless legs syndrome [30,31].
Sexual dysfunction
POI can affect sexual function, leading to decreased libido, sexual dissatisfaction, and dyspareunia. Estrogen deficiency, as well as psychological and social factors, contribute to these alterations. Psychotherapeutic support and both hormonal and non-hormonal treatments are recommended [32]. POI may also pose a risk for unplanned pregnancy, as HRT does not provide contraception [33].
Autoimmune diseases
POI may be a risk factor for autoimmune diseases such as rheumatoid arthritis and systemic sclerosis [34]. On the other hand, the autoimmune cause of POI may be related to comorbidities such as cardiovascular events and osteoporosis [25,35].
Mortality and additional morbidities
POI increases all-cause mortality, particularly from cardiovascular causes [35,36]. Studies indicate a higher risk of chronic kidney diseases and increased mortality in women with POI [37]. HRT treatment may mitigate some of these risks, and in cases of Turner Syndrome, higher mortality and morbidity rates are observed, making early diagnosis and treatment beneficial for these patients [38-41].
Diagnosis of POI
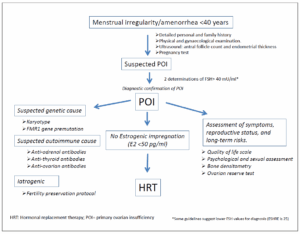
The diagnosis of POI is recommended for women under the age of 40 who present with secondary amenorrhea or oligomenorrhea lasting at least three months. The protocol advisable in POI diagnosis is detailed in Table 2 which summarizes the modified guidelines of the AEEM consensus published in 2015 [1], the EMAS guidelines from 2019 [42] and the ESHREIMS 2024 [27].
Diagnostic criteria
Commonly, the diagnosis of POI is confirmed by an increase of FSH levels above 40 IU/L on two separate occasions. However, lower threshold values (25 IU/L) have been proposed as a part of the evaluation protocol [27].
Medical history and family background
Although most cases of spontaneous onset of POI are idiopathic and lack specific diagnostic markers, the diagnosis is based on clinical evaluation and non-specific laboratory assessments to define the condition and assess potential complications and reproductive consequences. Certain autoimmune diseases, surgical treatments, radiotherapy, chemotherapy, or endometriosis may predispose women to POI. Additionally, POI has been associated with exposure to endocrine disruptors, smoking, psychological stress, violence and general or ovarian-specific infections [43,44].
Pelvic ultrasound (Ovarian Reserve Assessment)
Follicle count and ovarian volume have been correlated with AMH levels; therefore, ultrasound assessment may serve as a substitute for AMH measurement in cases where it is not available.
Genetic testing
Karyotyping and screening for FMR1 gene premutations in peripheral blood are recommended. Karyotyping can rule out Turner Syndrome, while a repeat expansion of 55 to 200 CGG triplets in the 5’ region of the FMR1 gene suggests a genetic cause of POI [45].
Autoimmune Testing
Antibody testing should be considered in patients with a personal or family history of autoimmune diseases, particularly affecting the adrenal glands or thyroid. Due to the low specificity of anti-ovarian antibodies, their routine assessment is not recommended. Instead, testing for anti-thyroid and anti-adrenal antibodies is advised [12]. Infectious disease screening Microbiological cultures or serological tests should only be performed when an infectious etiology is suspected.
Assessment of possible complications
Given the endocrine, cardiometabolic, and skeletal impact of POI, evaluations should include thyroid and adrenal function tests, lipid profile, insulin resistance assessment, and bone densitometry. Additionally, a thorough evaluation of psychosexual health and quality of life is necessary for women with POI. Different validated scales or questionnaires can be used to assess mood and female sexual function disorders; the Cervantes Scale is one of the many used in this regard [46] (Figure 2).
Management of patients without reproductive desire
The first and most important measure recommended for all women diagnosed with POI is the promotion of a healthy lifestyle, as it can improve their quality of life and even increase overall survival rate. Hormonal treatment in women with POI is considered a replacement therapy. Therefore, in this condition, the term HRT is appropriate. Having diagnosed POI, it is recommended to initiate HRT as soon as possible and continue it until the physiological age of ovarian function cessation (50 years). In this context, the benefits outweigh the risks for most of these patients [27,42,47,]. Short treatment periods or delayed initiation have not demonstrated long-term benefits. One year of amenorrhea is not a prerequisite for starting a treatment [48,49].
The most significant benefits of HRT in POI patients include:
- Cardiovascular health: prevention of atherosclerosis and cardiovascular events [50].
- Bone health: prevention of fractures and bone mass loss [51].
- Dementia: women with POI who have not undergone HRT present a higher risk of developing neurodegenerative diseases such as Alzheimer’s disease [52].
- Mortality: reduced all-cause mortality risk, particularly from cardiovascular causes [53,54].
- Management of menopausal symptoms.
- Sexual development: in cases of primary amenorrhea, HRT facilitates the development of secondary sexual characteristics.
In the absence of risk factors or medical conditions contraindicating its use, the benefits of HRT significantly outweigh the risks, making the risk-benefit balance clearly favorable for women with POI [55]. Psychological support alongside POI diagnosis is highly recommended. Many women experience anxiety, depression, and low self-esteem following diagnosis, which should be considered when designing a comprehensive therapeutic approach.
1.Choice of hormonal treatment
The decision regarding HRT should always be shared with the patient and be based on accurate information, presented in simple, transparent terms. HRT containing estradiol, the natural estrogen, has been considered more effective for maintaining bone mass than ethinyl estradiol, the synthetic estrogen included in most contraceptives, particularly in low-dose formulations. However, recent studies have not confirmed definitive conclusions on this matter [56]. Therefore, other factors must be considered, such as the need for contraception, economic cost, safety, tolerability, and patient preference regarding withdrawal bleeding when selecting a treatment. Tibolone has not been studied in POI patients and is therefore not recommended until the physiological age of menopause.
2.Hormonal contraception treatment
The likelihood of spontaneous pregnancy in women diagnosed with POI with a normal karyotype is approximately 5–10% [57]. The use of HRT could theoretically increase the possibility of ovulation by decreasing LH and FSH levels, which may prevent premature luteinization of antral follicles, on one hand, and downregulate ovarian receptors on the other [58]. Many contraceptives include a hormone-free interval, during which women are left without hormonal treatment. Thus, regimen with shorter hormone-free intervals (4 days) may be considered [59]. Another hormonal contraception option is the use of transdermal or oral estradiol combined with an intrauterine system that releases 52 mg of levonorgestrel for up to 5 years, according to its technical specifications. The management of contraception should follow the same safety criteria that govern contraceptive use in the general population [60]. The transition to HRT can be made when spontaneous conception is highly unlikely, which should be evaluated individually and generally considered at least 2 years after the POI diagnosis [59].
3.Hormone replacement therapy (HRT)
a. Estrogens
When selecting estrogen therapy, the following considerations should be taken into account:
- In general, compounds containing estradiol, the naturally produced hormone, are preferred.
- The transdermal route of administration might be the preferred choice for safety reasons in some cases.
- The recommended doses are replacement ones; as such, the concept of “the lowest effective dose for the shortest duration” does not apply.
- Estrogens are administered as monotherapy in hysterectomized women, and in combination with progestogens in women with or without a uterus but in particular conditions (i.e. endometriosis, endometrial cancer).
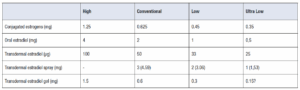
Replacement doses are defined as those achieving biologically equivalent estradiol levels of 50–100 pg/mL of circulating 17-β-estradiol. Despite individual pharmacokinetic variations, these levels are typically achieved with oral administration of 2–4 mg/day of estradiol or 0.625–1.25 mg of conjugated equine estrogens, transdermal patches releasing 50–100 µg of estradiol daily, 1.53-4.59 mg of estradiol spray or 1.5–3 mg of transdermal estradiol gel (Table 3).
b. Progestogens
Progestogen is included in HRT to counteract the endometrial-stimulating effects of estrogen, thus, considering this aspect, the right dosage of progestogen for a specific estrogen dose and regime must be chosen. Progestogens are classified into natural progesterone and synthetic progestins. Natural progesterone and dydrogesterone (not available in Spain) are associated with better glycemic profiles and improved metabolic, cardiovascular, and breast safety [61]. Most progestogens are administered orally; however, progesterone can also be administered vaginally. Another option is intrauterine administration via a levonorgestrel-releasing intrauterine system (52 mg), providing endometrial protection for up to five years, particularly beneficial for women transitioning to menopause with heavy menstrual bleeding.
c. Androgen treatment
There is increasing evidence that women with POI have lower androgen levels compared to age-matched controls. This could have an adverse impact on sexual function and may contribute to other health problems such as fatigue, decreased endurance, osteopenia, and sarcopenia [62]. A recent global consensus on testosterone use has confirmed that menopausal women receiving physiological doses of testosterone could experience significant improvements in overall sexual function and desire with minimal adverse effects [63]. Interestingly, clinical studies in patients with POI suggest that supplementation with dehydroepiandrosterone, either alone or in combination with estrogen-progesterone therapy, may enhance ovarian reserve and improve fertility outcomes [64]. However, real life treatment options to achieve physiological doses (5 mg/day) in clinical practice are very limited and off-label, making testosterone use very complex in these patients.
d. Alternative treatments for POI complaints
When HRT is contraindicated or the patient prefers not to use it, other options for symptom management are recommended. These include recently introduced neurokinin-3 receptor antagonists, as well as traditional treatments such as antidepressants, phytotherapy, and alternative natural therapies. The efficacy of these treatments in symptom relief is comparable to that observed in the general population; however, they do not provide the long-term benefits normally associated with HRT. Bisphosphonates should generally be avoided as a first-line treatment for preventing bone mass loss in a young population, as their long-term use is associated with reduced bone turnover, which may be detrimental to musculoskeletal health. However, bisphosphonates may be necessary when HRT is contraindicated or when BMD does not improve with hormonal treatment. Pivotal studies on different pharmacological options rarely include young patients. Therefore, in the absence of specific evidence, the recommendations for managing GSM in POI patients align with those for the general population [32,47].
Special situations
Treatment options for women with POI and reproductive desire
A POI diagnosis can be devastating for women, particularly those who have not completed their reproductive plans. Treatments using their own gametes have very limited success rates, leading most patients to consider oocyte donation, adoption, or choosing not to pursue treatment. It is essential to provide clear and realistic information regarding reproductive success rates for women diagnosed with POI. While spontaneous pregnancy is rare (5–10%), it remains a possibility, necessitating contraception for those who do not desire pregnancy. Fertility preservation after a POI diagnosis is ineffective. Leading medical guidelines to advise against recommending this approach. Instead, proactive fertility preservation, such as oocyte vitrification, should be considered in at-risk women before POI onset. The following groups should consider early fertility preservation
- Daughters of women with POI.
- Carriers of Fragile X premutations.
- Patients with Turner Syndrome mosaicism.
- Patients with autoimmune polyendocrine syndrome.
- Patients undergoing gonadotoxic treatments (chemotherapy, ovarian surgery, pelvic radiotherapy) [66].
For patients with established POI, ovarian stimulation for in vitro fertilization (IVF) using their own oocytes is rarely successful. Similarly, ovulation induction has not shown a significant increase in live birth rates. For highly motivated patients under 37, accumulating oocytes from stimulated or natural cycles may be considered [66]. Experimental treatments aimed at reactivating dormant follicles—such as stem cell infusion, intraovarian platelet-rich plasma injection, and mechanical follicular activation—may be viable options in selected cases, but not yet generalizable to all cases of POI. The most effective treatment remains oocyte donation, which is a well-established and safe technique. Except for cases involving uterine malformations (i.e. Turner Syndrome with uterine hypoplasia), pregnancy rates are very high. For patients requiring both egg and sperm donation, embryo donation is a valuable option.
Management of POI in cancer survivors and BRCA mutation carriers
Twenty-five percent of cancers in women are diagnosed before age 50, and their treatments often lead to POI. Symptoms and consequences of POI are often more severe in cancer survivors due to the abrupt and severe estrogen deficiency, concurrent androgen deficiency [67,68], and adverse effects of adjuvant therapies [69]. These patients require more complex care due to differences in risk profiles, self-perception, and limited safety data regarding HRT. Nonetheless, many women survive or live with cancer and may experience the consequences of past or ongoing treatments, including menopausal symptoms, increased risk of chronic diseases, psychological and social challenges, and diminished quality of life and sexual function. Managing these patients requires symptom relief, quality-of-life preservation, and prevention of hormone deficiency-related morbidity and mortality [70]. Women with BRCA mutations who undergo risk-reducing bilateral salpingo-oophorectomy also develop POI and face increased long-term morbidity and mortality if estrogen deficiency is not addressed. The primary consideration when managing these patients is the safety and eligibility of HRT [71,72], which remains contraindicated or uncertain in some cases but fully indicated in others [73-75]. General recommendations include:
- At cancer diagnosis and treatment initiation – Provide support and discuss non-pharmacological strategies.
- After initial treatment (disease-free patients) – Assess quality of life, health risks, prognosis, tumor type, adjuvant therapy, age, medical history, and POI-related health risks. Consider HRT if appropriate. If contraindicated or declined, explore alternative treatments.
- For hormone-dependent cancers (i.e. luminal breast cancer or type 1 endometrial cancer) – HRT is contraindicated during breast cancer adjuvant therapy and for at least two years in endometrial cancer. If HRT is not an option, standard alternatives should be used. Surveillance of endometrial health in patients taking tamoxifen and initiation of bone-protective therapy in patients on aromatase inhibitors should be applied. Vaginal estrogen and prasterone may be safe options for vaginal atrophy [76]; however, patients are rather reluctant to follow this type of treatment. Laser and radiofrequency treatments are available but lack long-term efficacy and safety data. After completing adjuvant therapy, ospemifene is an oral option for treating vaginal atrophy [77].
- For non-hormone-dependent cancers – HRT is the firstline treatment for POI, even in asymptomatic patients. The choice of preparation, regimen, and dosage should be tailored to the patient’s needs, their medical history and risks involved. Treatment should continue at least until the age of 50.
- For patients with metastatic disease – Increasingly longer survival rates necessitate a focus on symptom relief and quality of life, with treatment decisions tailored to disease prognosis.
Women with BRCA mutations share psychological concerns [78], including cancer phobia and fear of hormone therapy. Despite their increased cancer risk, bilateral salpingo-oophorectomy reduces breast cancer risk by 50%, and HRT does not appear to alter this risk [79]. Although evidence remains limited, no studies indicate that HRT increases breast cancer risk in these women [80]. Additionally, HRT recommendations should not differ from those for the general population. Offering HRT before prophylactic oophorectomy increases patient acceptance and optimizes long-term morbidity and mortality outcomes, particularly in mastectomized patients [79,80].
POI information for POI patient
Women diagnosed with POI should receive information about their condition that is tailored to their level of understanding in order to facilitate shared decision-making regarding the treatment and follow-up [76]. Essential information should include:
- Pathophysiology: POI occurs when the ovaries stop functioning properly before age 40, leading to reduced hormone production (estrogen, progesterone, and androgens) and affecting fertility.
- Causes: Many cases are idiopathic (unknown cause), though some result from genetic, medical, surgical, or environmental factors.
- Symptoms: Common symptoms include hot flashes, night sweats, sleep disturbances, and vaginal dryness.
- Reversibility: Unlike menopause, POI is not always permanent. Menstruation may occasionally return, and spontaneous ovulation and pregnancy occur in 5–10% of cases.
- Bone health risks: POI increases the risk of osteoporosis and fractures, making preventive bone health measures crucial.
- Cardiovascular risks: POI is associated with higher cardiovascular risk, requiring lifestyle interventions for prevention.
- Fertility considerations: POI can be emotionally distressing for women planning to conceive. Ovarian function and ovulation can be unpredictable. Assisted reproductive technologies, such as oocyte donation, may be an option.
- Treatment adherence: Long-term medical follow-up and treatment are necessary to manage symptoms, protect bone and cardiovascular health, and provide emotional support.
- Doctor-patient relationship: Open communication with healthcare providers helps ensure adherence to treatment and holistic management of this life-altering condition.