Introduction
The optimization of frozen embryo transfer (FET) protocol has yet to reach a consensus amongst in-vitro fertilization (IVF) clinicians. Natural cycle FET and artificial/hormone replacement FET cycles have reported to have similar pregnancy and live birth outcomes. Differences in protocols lie in the use of different progestogens and /or human chorionic gonadotrophin (hCG), by different centres. The use of dydrogesterone (DYD) in luteal phase support of natural FET cycles has also not been well documented.
Methods
A retrospective analysis of 893 FET cycles from January 2014 to December 2019 was made from a public hospital’s single in vitro fertilization (IVF) centre out of which 364 were natural FET and 529 were artificial cycles. Institutional Review Board (IRB) approval was not required for the clinical audit of retrospective data which was performed to improve existing clinical practices in our hospital and permission was not sought in this study. FET cycles from preimplantation tested embryos, donor oocytes and donor embryos were excluded from the analysis.
For natural cycle FET protocol, pre-follicular letrozole for enhancement of ovulation was given for 5 days as 2.5 to 5 mg daily dose from days 2 or 3 of menstrual cycles in 50% of natural analyzed FET cycles. Oral DYD of 30 mg daily was started and used exclusively as the progestogen in the luteal phase support of all analyzed frozen thawed FET natural cycles HCG 5,000 IU was used as trigger (36%) and smaller doses of 1,500 IU as one or two injections in the first half of the luteal phase (96%). Embryos were obtained from IVF cycles or intracytoplasmic sperm injection, vitrified, and warmed for embryo transfer (ET). All ETs were performed with ultrasound guidance. Transfers were done from days 2, 3, 4 and 5 of the FET cycles. Day 6 vitrified embryos were transferred on day 5. One to three embryos were transferred in each cycle. Only grade 1 or 2 embryos were frozen, warmed and transferred.
A vaginal ultrasonographic scan was performed once for each woman on cycle days 10 to 14. On the day that the leading follicle had reached 14 mm in diameter, women started twice daily serum luteinizing hormone (LH) measurements to calculate the beginning of LH surge. DYD was started from the fourth day of detected LH surge corresponding to day 2 of embryo development. The embryo/embryos were warmed according to the age of embryo when it was vitrified and embryo transfer was performed on the planned date or cultured to the planned date. ET was performed with the help of trans-abdominal ultrasound. Luteal support with hCG was given up to the first half of luteal phase, when ordered by the clinician.
For modified natural cycle FET (mNC-FET), hCG 5,000 IU was administered when the leading follicle was 17-18 mm in diameter and ET was calculated according to the day of FET. DYD was started on the fourth day of hCG injection corresponding to day 2 of embryo development. DYD was continued till at least 10 weeks pregnancy and ultrasound viability scan was performed at 6 weeks. Luteal support with hCG was given as described before. There was no significant difference in outcome amongst the different protocols in the natural cycle FETs.
Three hundred and sixty-four cycles using oral DYD with hCG were used in this analysis. The small number of cycles with DYD alone for luteal support were excluded (6%). In the artificial cycle, GnRH agonist was used to suppress menstrual cycles in 133 of cycles and serum estradiol levels were checked after 2 weeks and estradiol administration was initiated orally with estradiol valerate at a 6 mg/day dose when the serum levels were in the pre-follicular phase.
In 399 artificial cycles, estradiol valerate was started on the second or third day of the menstrual cycle. After 14 days of estrogen therapy, a transvaginal ultrasound was performed to assess the endometrial thickness. If the thickness of the endometrium was < 7 mm, estrogen therapy was extended for another seven days, and the dose was reviewed and sometimes increased to 8 mg/day. When a 7-mm thick, triple-line endometrium was observed, micronized vaginal suppositories (MVP), 400 mg three times daily was started. ET was performed according to the day of warming of the vitrified embryos calculated from the initiation of progesterone pessaries.
Supplementation with estrogen and progestogen was continued at the same dose until the pregnancy test, performed 15 days after ET. This support was continued up to 10-12 weeks in viable pregnancies. Ultrasonography was performed during the sixth week to confirm clinical pregnancy.
Statistical analysis
Categorical variables are presented as numbers and percentages. Continuous variables are displayed as mean with standard deviations. Chi-squared test was used for the comparison of categorical variables and a T-test for continuous variables. Chi-squared test for proportions compared the rates between two groups, accounting for different numerators and denominators (embryos). Statistical significance was set at p value of < 0.05.
Epidemiology
Mean age of women in the natural cycle FET group was significantly higher compared to the artificial group (36.5 vs 35.2 years, p<0.05). When comparing indications for initial IVF in the 2 groups, it was found that there were higher number of women with polycystic ovary syndrome as a diagnosis in the artificial group compared to the natural cycle group (11% vs 3%, p=0.0002). This could explain the younger age group in the artificial FET cycle. The numbers of all other indications for IVF between the 2 groups were not significant. Male factor diagnosis contributed to almost half of the indications for IVF in the 2 groups (43% artificial vs 48% natural, non-significant).Unfortunately, variables like weight, basal metabolic index and previous reproductive history were not available for analysis
Artificial FET cycle: variable of suppression versus no suppression
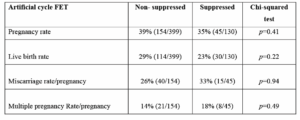
There was a total of 529 ETs using the analyzed artificial FET cycles. Suppressed cycles with GnRH agonist and estradiol valerate and progesterone pessaries were conducted in 130 cycles and those without suppression with only estradiol valerate and progesterone pessaries was 399 cycles. The success rates were compared and although the miscarriage rate appeared to be higher in the artificial suppressed cycle group, there was no statistical significance (Table 1). The subgroups of artificial cycle FET were combined and used for analysis.
Total FET outcomes
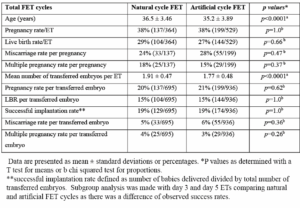
There was a total of 364 FET natural cycles using DYD with hCG for luteal support. Mean age was 36.5 years. Pregnancy rate per ET was 38% and LBR per FET was 29%. Miscarriage rate per pregnancy was 24%. Multiple pregnancy rate per pregnancy was 18%.
The statistics above were compared with 529 cycles of artificial FET cycles during the same period which used estradiol valerate and progesterone pessaries for luteal support.
Mean age of the artificial cycle FET group was 35.2 years, significantly lower than the natural cycle group. The pregnancy, live birth rates were similar between the 2 groups, but miscarriage rates trended to be higher in the artificial FET cycle group. Multiple pregnancy rate trended to be slightly higher in the natural cycle FET group. However, this did not reach statistical significance. Mean number of transferred embryos was significantly higher in the natural cycle FET. The per embryo statistics were calculated to negate the influence of the difference in the number of transferred embryos. Results between the 2 groups were very similar and not significant (Table 2).
Day 3 ET outcomes
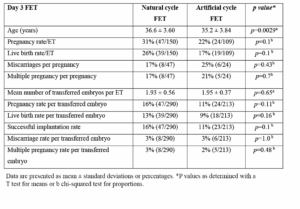
Day 3 ETs showed a similar significant age difference as in the total FET statistics. Despite an older age cohort, day 3 natural FET cycle had a higher pregnancy, live birth rates and lower miscarriage rates than day 3 artificial FET cycles but there was no statistical significance. There were lower but non-significant multiple pregnancy rates in the natural cycle FET group. Mean number of transferred embryos per ET was not significantly different (Table 3).
Day 5 ET outcomes
Twenty one percent of day 5 FETs were natural cycle FET and 79% were artificial FET cycles. Again, women from natural FET cycles were older compared to those of the artificial cycle group. There was a greater observed age gap (36.9 versus 34.9 years). Trends were higher in success rates, but pregnancy, live birth and miscarriage rates were not significantly different.
There was an unexpectedly higher multiple pregnancy rate per pregnancy on day 5 natural FET cycles. This could be explained by more embryos being transferred per cycle in the natural cycle FET group. However, when per embryo statistics were looked at, multiple pregnancy rate per embryo and successful implantation rate (baby per embryo) were significantly better in the natural cycle D5 transfers compared to the artificial cycles. Another explanation would be better embryo utilization and implantation which manifested in increased multiple pregnancies in day 5 of natural FET cycle (Table 4).
Discussion
DYD is a retro-isomer of progesterone which was introduced in 1961 for medical use. It has been widely used for luteal and early pregnancy support [1-3]. Its use in IVF was established scientifically in 2017 following the publications from the LOTUS I and II trials [4,5]. The drug has advantages of having good oral bioavailability and is well tolerated by patients. DYD also appears to have no affinity for androgenic, estrogenic, glucocorticoid or mineralocorticoid receptors [3], demonstrating a favourable safety and tolerability profile in pregnancy, both for the mother and child {1,2]. Other used progestogens require intramuscular or subcutaneous injections or transvaginal route applications. Side effects include painful skin lesions and irritating vaginal discharge. In terms of FET cycles, DYD has been compared with other types of progesterone supplementations in the luteal phase in artificial FET cycles. Rashidi et al. [6] compared oral DYD, intravaginal suppository and intramuscular progesterone support in the artificial FET cycle. Their results showed similar clinical pregnancy and live birth rates; therefore, Rashidi suggested that using DYD in FET cycles should be implemented due to its ease of use, lower cost and higher patient satisfaction.
Few studies have reported successful outcomes with DYD in natural FET cycles [7-10]. The systematic review and meta-analysis of the effect of progesterone supplementation for luteal phase support in natural cycle FET cycles [11] concluded that there was moderate-quality of evidence suggesting that progesterone supplementation for luteal phase support was associated with increased live birth and clinical pregnancy rates in natural FET cycles compared to cycles without progesterone. Progesterone supplementation was associated with a higher live birth and clinical pregnancy rates in true natural cycle FET (tNC-FET) cycles. However only one study in the analysis used DYD for luteal support.
Progestogens can also be combined with hCG for luteal support in natural cycle FET. Tu et al. [12] showed that high doses of exogenous hCG showed a luteotropic effect on the human corpus luteum mainly manifested in increased serum progesterone levels and prolongation of the menstrual cycle but there was no effect on hormonal blood levels in early human gestation from 5 to 10 weeks. HCG has a long half-life of up to 5 days.
The meta-analysis of Mizrachi et al. [13] of over two thousand records showed that progesterone for luteal phase support improved live birth rates to hCG luteal support. The retrospective study of Wen et al. [14] showed that hCG did not improve clinical pregnancy and live birth rates compared to a control group of progesterone luteal support and no hCG in frozen-thawed blastocyst transfers. There was a consensus that hCG was not required in luteal support. Our study included DYD and hCG for luteal support as numbers of luteal support with DYD alone were small. Hence the question as to whether hCG was required for luteal support remains unanswered.
Zarei et al. [15] carried out a randomized controlled trial comparing four luteal phase support regimens including vaginal progesterone 400 mg/day, DYD 20 mg/day, a combination of DYD 20 mg/day and gonadotropin-releasing hormone agonist, and a combination of DYD 20 mg/day and hCG. Their results showed that the DYD-only group had a lower clinical pregnancy rates than the other three groups. There were no significant differences among the four groups in terms of the ongoing pregnancy rate or miscarriage rate. The authors’ hypothesis regarding the low pregnancy rate when using DYD alone was the reduced dosage of DYD of 20 mg/day compared to a dosage of 30 mg/day. Our study used 30 mg daily of DYD.
DYD and hCG have been used in our centre for the past 30 years in assisted reproduction programmes; although DYD use would has been currently considered as off-label. Hence, we can report results from 2014 onwards. There was no comparison between oral DYD and vaginal progesterone for luteal support in our cohort of natural FET cycles as DYD was the exclusive progestogen used. Natural cycle FET was compared with artificial cycles using estradiol valerate and progesterone pessaries. Oral DYD was given as 30 mg daily, an adequate dose as used by most centres.
Age was consistently significantly higher in the natural FET cycle group compared with artificial cycles by 1.3 years. This could be explained by a higher number of women with polycystic ovary syndrome as diagnosis in the artificial FET cycle group. They are usually younger and present with anovulation and are more likely to require artificial FET cycle treatment.
Despite that, there were no differences in pregnancy, live birth, miscarriage and multiple pregnancy rates in total FET results. There was a significantly higher mean number of transferred embryos per ET in natural cycles compared to artificial cycles in the overall statistics but pregnancy, live birth, miscarriage and multiple pregnancy rates were not significant (Table 2)
Natural cycle FET on day 3 showed non-significant higher pregnancy and live birth rates and lower miscarriage rates. Mean number of transferred embryos was similar between the 2 groups. There was no difference in the per embryo statistics in pregnancy, live birth, miscarriage and multiple pregnancy rates (Table 3).
Numbers of day 5 artificial cycles were more than natural cycles. There was a greater difference regarding age and mean number of embryos per ET between the 2 groups. Interestingly, multiple pregnancy rates were significantly higher in the natural cycle group and this could be due to more embryos being transferred. However, multiple pregnancy per embryo and successful implantation rate also increased significantly in the natural cycle day 5 FET implying better embryo utilization and implantation (Table 4). Increased age in the day 5 FET natural cycle did not reduce the multiple pregnancy rate. This finding should signal a change of policy to allow more single ETs amongst the older age group.
As expected, overall day 5 FET successful outcomes were better than day 3 FET. There was a trend in improved implantation (14%) in the natural FET cycle group between day 3 to day 5. Day 5 FET artificial cycle successful results improved dramatically compared to day 3 FET artificial cycle results. Implantation rates also improved (11%) but still lower compared to natural FET cycles.
No difference in successful outcomes has been observed between the 2 groups, natural versus artificial in previous studies [8,9]. However, implantation rates were not calculated in one study and per embryo statistics were not presented. Our retrospective study showed interesting results in subgroups of day 3 and day 5 transfers. It should be agreed that progestogen is needed for luteal support of natural cycle FET. In our study, DYD with hCG, used as luteal support er in natural FET cycles, showed best implantation results in day 5 transfers.
Further prospective controlled randomized studies with adequate numbers and better control of confounding variables are warranted.
Limitations
Our audit is limited by the presence of many variables. Heterogeneity cannot be controlled well in a real-world retrospective audit. However, this study presents real world data of the use of DYD over 6 years of luteal support. Demographic factors such as weight, basal metabolic index, reproductive history were however not available for analysis. Other unknown variables may have been present that may have influenced differences of results.
Conclusion
Historical and real-world data in our centre showed similar overall successful outcomes between natural cycle FET and artificial cycle FET over 6 years of analysis. Day 5 natural cycle FET with DYD and hCG luteal support resulted in significantly better implantation as reflected in higher multiple pregnancy rates per embryo and successful implantation rates per embryo compared to day 5 artificial cycle FET.