Introduction
Mature cystic teratoma (MCT) is one of the most common benign ovarian neoplasms, accounting for 10 to 20% of all ovarian tumors. It is typically located in the gonads, though it can also be found in extragonadal sites such as the brain, omentum, retroperitoneum and mediastinum [1,2]. Most cases are unilateral, but approximately 12% are bilateral. In unilateral cases, MCTs occur more frequently on the right side (72.2%) [3].
Ovarian teratomas are classified into three types: mature, immature, and monodermal (highly specialized, such as struma ovarii, carcinoid tumors, and neuroectodermal tumors). Mature teratomas, also known as dermoid cysts, account for 95% of all teratomas. They grow slowly at 1.8 mm/year and are usually large at the time of diagnosis [3].
Histologically, MCTs include at least two layers of well-differentiated mature germ cells (ectoderm, mesoderm, and endoderm). The most common tissues found in MCTs are skin and hair (derived from the ectoderm) and fat and muscle (derived from the mesoderm). When ectodermal tissues predominate, these teratomas are called dermoid cysts [3], but fat is present in more than 93% of cases.
MCTs are often asymptomatic and are detected incidentally during routine pelvic examinations, imaging studies, or abdominal surgeries for other reasons, such as cesarean delivery. However, about 20% of patients with MCT may present acute or subacute complications, including torsion, rupture, infection, autoimmune hemolytic anemia, paraneoplastic syndrome, or malignant transformation. Malignant transformation is rare (0.2–1.8%), and the neuroectodermal component is responsible [1-3].
Due to its histology, several tumor markers, such as CA-125 and CA19-9, may be elevated in MCT. Specifically, CA 19-9 is suggested as a potential marker of MCT. Additionally, the magnitude of elevation may serve as a warning of possible acute complications. Although it was once believed that significant elevations in CA 19-9 could indicate malignant transformation, this is no longer considered valid.
We present a clinical case of a patient with acute abdominal pain with significantly elevated CA 19-9 levels, associated with an elevation of CA-125 and ultrasound features suggestive of complicated MCT. We briefly review the characteristics of MCTs and the significance of the exceptionally elevated tumor markers, which are rarely reported in the literature.
Case report
This is a 35-year-old patient with nonspecific abdominal tenderness and dyspareunia lasting for one year. She had a non-notable medical history, and regarding her gynecological history, she was nulliparous, with normal periods, and condoms was her used contraceptive method.
The patient came to the emergency room with intense, acute abdominal pain. Upon examination, intense pain was noted in the lower abdomen, without signs of peritoneal irritation. Abdominal ultrasound revealed a left ovary tumor with an appearance suggestive of MCT. Serum analyses, including tumor markers were normal. The patient improved with analgesia and was discharged.
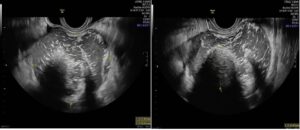
A week later, the patient visited our gynecology outpatient clinic, reporting intermittent abdominal pain. Gynecological examination revealed a tumor that bulged the anterior uterine and left adnexal area, which was painful and had an elastic consistency. Vaginal ultrasound showed a normal uterus with secretory endometrium, a normal right ovary, and a tumor with heterogeneous content in the left adnexa (8.8 x 7.8 x 5.8 cm), with scattered hypoechoic dots, and a dense central formation approximately 3.3 cm in size (Rokitansky nodule). The edges were thickened, with three anechoic chambers containing some papillary formations but no Doppler signal. A small amount of ovarian parenchyma was observed at the periphery, with a modest amount of fluid in the left perianexal region and Douglas space (Figure 1). At this point, CA 19-9 was markedly elevated to 3,911.77 U/mL (previously 33 U/mL), and CA-125 was elevated to 141.20 U/mL (previously 8 U/mL).
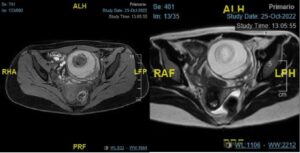
A pelvic CT scan confirmed the left adnexal mass suggestive of an ovarian teratoma with a central fatty nodule (Rokitansky nodule) and calcifications in some septa and along the periphery of the tumor. And pelvic MRI reported a left adnexal mass measuring 7.4 x 8.8 x 6.7 cm, with heterogeneous signal on T2 and T1 sequences, hyperintense component suggesting hemorrhagic content, and a 3.3 cm central nodule (Figure 2). There was marked thickening of the left ovarian vascular pedicle, with engorged vessels contacting the posterior and superior margins of the lesion, raising suspicion of torsion.
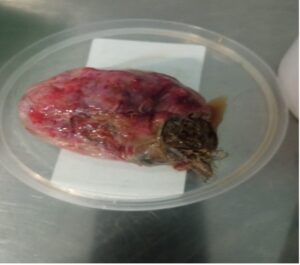
Two weeks later, CA 19-9 levels remained elevated but decreased to 2,248.68 U/mL, while CA-125 normalized at 32.3 U/mL. We concluded that it was a left ovarian teratoma with partial torsion, and surgical options were considered, prioritizing the risk of recurrent torsion but keeping in mind the probably benign condition of the tumor. In view of elevated tumor markers and that some areas of the tumor suggested solid component, we thought diagnosis was benign with changes due to partial torsion, and managed accordingly. In any case, the patient preferred removal of the left ovary and laparoscopic surgery was performed, removing a twisted left adnexal tumor measuring approximately 8 x 6 cm (Figure 3). Histology confirmed the diagnosis of MCT.
Discussion
MCT is a common entity in gynecological practice, which is generally well recognized and not concerning due to its benign nature. However, sometimes this is not so clear; moreover, if not removed, complications can arise and may require surveillance and early detection.
While ultrasound is the most common tool for MCT diagnosis and is sufficient, the features of the tumor are not always specific enough to confirm the diagnosis. Studies indicate that up to 30% of MCTs may not be visible on ultrasound due to associated pelvic abnormalities (i.e. endometriomas, large fibroids, or large contralateral ovarian masses). In some cases, there may even be doubts about an associated malignant process [1,4,5]. In non-diagnostic ultrasound cases, a CT scan can effectively detect fatty and calcified components of MCTs with a sensitivity of 93–98%, and it provides valuable information about the mass's relationship with surrounding tissues. Similarly, MRI has high sensitivity in detecting fat and calcifications, so it can be an even better alternative tool in cases where ultrasound findings are inconclusive [2]. The different combinations of mature tissue with distinct arrangements inside the MCT cause a wide spectrum of radiological presentations, ranging from a purely cystic mass to a complex cystic mass with relevant solid component [3,4].
Serum CA 19-9 levels can also be useful in diagnosing MCTs. Introduced by Koprowski et al. in 1979, who described a monosialoganglioside antibody linked to an antigen found in cultures of colorectal cancer cells and in other gastrointestinal neoplasms. CA 19-9 is primarily used as a marker for gastrointestinal malignancies, especially pancreatic carcinoma. In this context, levels greater than 300 U/mL are predictive of malignancy [6-8]. CA 19-9 can also be elevated in benign conditions such as pancreatic-biliary diseases, liver disease, thyroiditis, diabetes, autoimmune diseases, and gynecological conditions like endometriosis and mucinous ovarian tumors [9].
Although the simultaneous elevation of CA 19-9 and CA-125 have been associated with malignant mucinous ovarian tumors, both markers can be elevated in MCTs. In any case, CA 19-9 is more frequently elevated and found elevated between 39-59% of MCT cases. Of course, this marker alone is not sufficient for the diagnosis of a MCT, but if the nature of the pelvic mass cannot be determined by imaging tests, CA 19-9 may be of some value for MCT suspicion [1]. It is argued that in cases of isolated elevation of CA 19-9, the suspicion of ovarian malignancy might be low [2,9], but high levels introduce logical concern.
The elevation of CA 19-9 correlates with important fatty and dental components in MCT. Literature reports suggest a correlation between elevated CA 19-9 levels and a large tumor diameter, increased rate of ovarian torsion, and cyst rupture. Therefore, it has been proposed that in those cases of high clinical suspicion with marked elevation of CA 19-9, an early diagnosis of MCT complicated with torsion can be made, and therefore a prompt surgery is necessary. Some studies have also suggested a link between CA 19-9 and bilateral MCTs, although this has not been conclusively demonstrated [2,9-11]. The highest CA 19-9 levels reported in the literature in MCT cases have been 25,590 U/mL [8,12]; however, these extreme elevations are rare, and levels above 1,000 U/mL are uncommon [9]. Therefore, our case with an elevated CA 19-9 value of 3,911 U/mL is rare in routine clinical practice.
The precise pathophysiological mechanism behind CA 19-9 elevation in MCTs remains unclear. Elevated levels of CA 19-.9 are known to be present in the intracystic fluid of MCTs, which is secreted from the epithelial lining of the cyst wall, suggesting that the possible mechanism would be leakage of the cystic fluid. This could explain the significant CA 19-.9 elevation due to torsion that produces necrosis and wall micro-perforation, with dissemination of the cystic component of the MCT [2,6,9], leading to an abrupt and marked increase in CA 19-9 serum concentrations. This may introduce some concern about possible malignancy, but a conservative approach may be justified, although in our patient, it was not possible because of the patient preference.
In conclusion, MCT is a common benign adnexal pathology in gynecological clinical practice that, in general, is diagnosed quite accurately with clinical data and imaging tests, but in some cases with diagnostic doubts, CA 19-9 levels can be considered a potential indicator that can help to discern possible acute events, which could require prompt surgical treatment with inestimable benefits for the patient; and, in such circumstances are not necessarily related to malignant disease.