Introduction
In 2020, breast cancer became the most commonly diagnosed cancer type in the world with more than 2.26 million new cases and almost 685,000 deaths worldwide, being the most common cause of cancer death in women, and the fifth overall [1]. For breast cancer patients, chemotherapy (CT) is typically administered after surgery to prevent metastasis, as the first treatment option to reduce tumour size, or to struggle against metastasis in advanced breast cancer. Taxanes, doxorubicin and epirubicin are the most frequently used drugs, especially in the early stage of breast cancer disease [2]. Sometimes, other drugs such as fluorouracil (5-FU) and cyclophosphamide (Cytoxan) could be used in a combined CT regimen. Actually, adjuvant and neoadjuvant treatments are more effective when different drugs are combined. The most frequent CT combination for breast cancer is FEC (Fluorouracil, Epirubicin and Cyclophosphamide), although nowadays targeted cell therapy plays an important role as well.
CT, despite being very effective against malignant cells, can also cause damage to normal cells. Well-known oral adverse effects are oral mucositis or stomatitis (inflammation and ulceration of oral mucosa), candidiasis [3], neurotoxicity, oral discomfort and pain, bleeding, dryness (due to salivary gland hypofunction) [4], taste disturbance, and increased susceptibility to bacterial, fungal and viral infections [5]. Mucositis appears in about 40% of patients receiving CT. Half of them may develop serious wounds requiring CT schedule modification, changes in oral intake or parenteral analgesic treatment [6]. However, there is scant data on CT adverse events affecting oral mucosa in breast cancer even though the number of breast cancer patients undergoing CT is increasing [7].
In short, the aim of this review is to highlight the impact of oral mucositis induced by CT in breast cancer patients regarding its occurrence, effect on quality of life, continuation of anticancer therapy as well as to underline possible treatments to prevent and manage it given that oral mucositis is a common symptom that is not always addressed correctly.
Methods
Studied condition
Oral mucositis induced by antineoplastic drugs, a major side effect of cancer therapy, is an inflammation and ulceration of the oral mucosa. It usually appears as a reddish lesion that causes burning or in the form of ulcers throughout the mouth.
Highest prevalence of mucositis is observed among patients treated with aggressive CT (cyclophosphamide and methotrexate). The intervals between drug administration seem to be more important than total dosage [8], as the risk of developing oral mucositis increases with the number of CT cycles {9]. The presence of opportunistic pathogens in the oral flora may also affect treatment outcome. However, the actual involvement of host factors in mucositis may vary based on the exact radiotherapy and CT regimens used [10]. Mucositis is more severe when patients show aplasia, a lower kidney function, lower granulocyte recount and when they receive a combined therapy (radiotherapy and CT); it is also affected by kind of malignancy, age, oral status before CT, and possibly by smoking history. Poor oral health increases the individual risk of developing mucositis, as does low salivary production, existing mouth damage, impaired immune status or high endogenous levels of pro-inflammatory cytokines.
Clinical characteristics of mucositis
Symptoms are variable but, in general, mucositis is presented as erythematous and atrophic areas broadly associated with desquamative areas in the oral mucosa that may lead to painful ulcers. The first sign of mucositis is erythema, which appears 4-5 days after starting CT. Healing appears 2 weeks later. Patients usually have burn mouth symptoms and inability to eat spicy meals at this point. After 7-10 days of ending CT, ulcers appear which cause discomfort and may lead to diet modifications. CT induced mucositis takes place on the movable mucosa, rarely affecting the dorsum of the tongue, the hard palate or the gingiva [8].
Classification of mucositis / toxicity scale
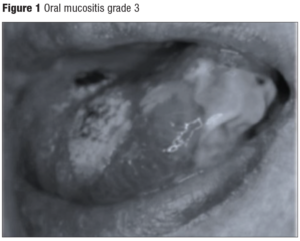
A universally accepted scale for the assessment of mucositis does not exist, but the two most commonly used scales are from the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), and that of the World Health Organization (WHO), which are detailed in Table 1. Nevertheless, Oral Mucositis Assessment Scales seem to be a more complete classification as the following items are described: presence of ulcers and erythema in both lips, both sides of buccal mucosa, tongue, floor of the mouth and soft & hard palate. Depending on ulcers and erythema severity, these are divided in 4 groups and 3 groups, respectively [11]. Therefore, this classification has also been included in Table 1. Figure 1 illustrates grade 3 oral mucositis according to the different available scales.
Search design
Literature search
An updated search was performed in PubMed and other data sources including The Cochrane Library, UpToDate, Embase, Web of Science, Scopus, Dialnet, and Cumulative Index to Nursing and Allied Health Literature (CINAHL) up to April 19, 2023. We used the following Medical Subject Headings (MeSH): “mucositis”, “oral mucositis”, “chemotherapy”, and “breast cancer”. To identify all of the articles that describe mucositis the following strategy was designed: mucositis AND chemotherapy AND breast cancer.
In order to have more specific results and to replicate the study, the final search was performed only in PubMed with the combination of terms “mucositis” AND “chemotherapy” AND “breast cancer”, choosing “in the last 10 years” (2013-2023) and “in the last 5 years” (2018-2023) filters with 265 and 125 results, respectively.
Results
Finally, a total of 125 studies were identified in the PubMed database selecting 89 for further examination by reviewing title and abstract content. After a two-step review, 65 articles met the inclusion criteria: evidence on oral mucositis in breast cancer patients treated with different chemotherapy agents. Selected manuscripts were written in English.
Controlled, randomized trials, prospective and retrospective studies and systematic reviews that evaluated different features of mucositis were included. Those articles that only focused on oral mucositis caused by radiotherapy or in other types of cancers were excluded, as well as duplicated articles and single case-reports. Of the reviewed articles, those which combined CT with bisphosphonates or osteonecrosis were excluded, because the main goal of this study was to investigate oral mucositis caused by CT.
Pathogenesis of mucositis
Mucositis is a multifactorial disease affecting 20-40 percent of patients receiving conventional-dose cytotoxic CT [10]. Oral mucosa injuries caused by chemotherapy are the result of a biochemical complex reaction [12].. Mucositis is due to direct inhibitory effect in deoxyribonucleic acid (DNA) replication and cell proliferation caused by CT, that reduces the renovating capacity of mucosal epithelium basal layer making, resulting in atrophy, collagen collapse and ulceration. As cell replication is higher, oral mucosa is greater affected [11]. Several chemotherapeutic agents are known to induce mucositis such as methotrexate when administered in dosages of 1 g/m2 or higher, procarbazine, cytarabine, doxorubicin, daunomycin, etoposide, and 5-fluorouracil [11].
Oral mucositis could be a very negative and often unexpected experience for cancer patients. Cell replication and maturation are affected by CT provoking changes in oral mucosa. Thus, basal layer loses its regenerative ability, producing erythema and sensitivity. Later, epithelium flaking, soreness, and tissue ulceration appear. These changes take place after 5-7 days of starting CT and in non-immunosuppressed patients they disappear after 2-3 weeks. Hemorrhage can occur if platelets decrease 25,000 units. Ulcers may be a portal of entry for bacteria that can cause a systemic infection leading to sepsis [11,13].
It is assumed that mucositis is a biological process of four phases: inflammatory phase; epithelial breakdown phase; ulcerative phase and healing period. The third phase is usually the most symptomatic with the greatest effects over the oral cavity, affecting the patient's quality of life the most. A collapse in a patient’s mucosal barrier occurs, and the combination between neutropenia and myelosuppression that likely coincide when receiving CT puts the patient at risk of oral cavity infection [6,8,10,13,14]. Eventually, during the last phase, re-population of the basal region of the epithelia can initiate from around 3–5 days after therapy and proliferation slowly but progressively increases with time, restoring a healthy functional mucosa rapidly as long as further trauma ceases [15].
Consequences of mucositis
Mucositis limits food consumption due to pain and discomfort to chew and/or swallow. It produces atrophy in chewing muscles and reduces intestinal absorptive surface, therefore increasing the risk of malnutrition [6,11]. It also produces difficulty with speech. Such adverse effects can significantly affect patient weight, mood, and daily functioning.
Mucositis is associated with increased morbidity and mortality besides significant additional hospital costs [4,14]. CT-induced myelosuppression places patients at significant risk of bacteraemia and sepsis from oral microorganisms resulting in increased days of fever, antibiotic use and hospitalization [8], with a relative risk of septicaemia four times greater than that of individuals without mucositis [9]. In addition, severe oral mucositis may interfere with the ability to deliver the intended course of therapy, leading to significant interruptions in treatment, and possibly impacting on local tumour control and patient survival. Quality of life of patients with mucositis is impaired, and the associated oral symptoms may exert a negative influence on patients’ physical tolerance to treatments or their attitude towards receiving further courses of CT [6,10,13]. Patients’ quality of life is impaired due to pain, so is their sociability due to talking difficulty, inability to swallow and mood changes. Modulation of the treatment regimen (use of lower doses or long recovery intervals between doses) remains the most effective means to limit incidence and severity of mucositis. Nonetheless, this reduces the efficacy of anticancer therapy [10].
Management
We have analyzed 65 studies regarding CT-induced mucositis in a specific population. There are few studies about mucositis in breast cancer, which is paradoxical considering the high incidence of this cancer in women.
The first study is that of McCarthy et al. [5] which has a sample size of 34 women undergoing treatment with cyclophosphamide 80 mg/m², methotrexate 35 mg/m², 5FU 500 mg/m², and vincristine 1 mg/ m². Out of these 34 women, 22 experienced any symptoms, 19 mucosal pain, 1 numbness, 3 paresthesia. This was the first study that positively correlates oral mucositis with CT for breast cancer, but its sample size was too small [5].
In 2003, Chan et al. [13] also studied oral mucositis in a population of 94 Chinese women receiving CT, but of these 94 women only 4 were suffering from breast cancer. In this study, 5% of women suffered from any degree of mucositis. The main problem of this study was again sample size: it only studied 4 women with breast cancer comparing mucositis among all cancers without differencing them neither specifying CT doses.
In 2008, Jensen et al. [4] also conducted a study on oral mucositis and breast cancer. This was a case-control study with 45 women with breast cancer receiving CT and 31 women not receiving CT (only treated surgically.) CT was cyclophosphamide 600 mg, epirubicin 60 mg and 5-FU 600 mg. For postmenopausal women, Epirubicin was changed for methotrexate 40 mg. The study concluded that adjuvant CT in breast cancer patients causes mucosal lesions, oral candidiasis and taste disturbances. The main limitation of this study was that it did not make any differences between patients with oral removable prosthesis and the rest of patients because such situation can per se cause candidiasis and taste disturbances.
Napeñas et al. [16], in 2010, conducted a study to assess microbiological changes in 9 patients with breast cancer providing data on oral mucositis. Patients underwent CT regimens with adriamycin 60 mg/m² and cytoxan 600 mg /m². Six patients had grade 1 mucositis (soreness/erythema) and 3 grade 0. The limitation of the study was also its very small size.
Kimura et al. [17], in 2019, studied retrospectively cost-effectiveness and safety of palbociclib and everolimus for treating advanced and recurrent breast cancer (13 vs. 22 patients). In patients receiving palbociclib, stomatitis was very low compared to everolimus group (7.7 vs. 77.3%). Discontinuation due to adverse events occurred in 5/22 everolimus-treated patients (23%) whereas all palbociclib-treated patients could continue with their treatment.
In 2021, Gadisa et al. [18] analysed the impact of toxicities provoked by adriamycin- cyclophosphamide [AC] and AC followed by paclitaxel [AC-T] regimens on quality of life among 100 women with breast cancer in Ethiopia. In this prospective study, oral mucositis of grade 2 and above was associated with significant impairment of physical and role functioning, and fatigue and financial difficulties of the patients. AC and AC-T regimens significantly deteriorated different quality of life items particularly during the first two cycles of CT.
In 2022, Schmidt et al. [19], designed the DESIREE trial which investigated the use of an everolimus dose escalation schedule (EVE esc) with the goal of reducing stomatitis and subsequently premature treatment interruptions or dose reductions. Within 12 weeks, the incidence of episodes of stomatitis grade ≥ 2 were significantly lower in the EVE esc arm compared to the EVE 10 mg arm (28.8% vs 46.1%; odds ratio 0.47, 95% confidence interval 0.24-0.92, P = 0.026). Authors concluded that this alternative strategy could be useful to reduce everolimus-related oral mucositis.
Discussion
The concern of CT-induced mucositis among health care providers dealing with breast cancer has increased notably from the first description by Hogan in 1951 [20]. At present, there are several reasons that may explain their interest on the topic. Firstly, the incidence of breast cancer is increasing sharply [21,22]. Considering that on average 20 to 40 percent of cancer patients may develop an episode of mucositis [7,23], this adverse event will have a huge impact on the health system. Secondly, mucositis not only affects patient’s quality of life causing not only inability to eat, but also impairs antineoplastic treatment as it is an adverse event impacting on patient's nutritional status which is considered a relevant prognostic factor [23,24].
Regarding preventive recommendations, besides establishing oral care protocols to prevent stomatitis across different therapeutical regimes, it has also been suggested that patients can rinse their mouths with a bland non-alcoholic sodium bicarbonate-containing mouthwash four to six times a day as a way to avoid mucositis. Moreover, alkaline rinses with other options are used as supportive treatments aimed at controlling symptoms [23].
Regarding treatment strategies, there are several options:
- Symptom control. Oral mucositis requires analgesia, physical activity, psychological approach and oral attention. Chlorhexidine, acyclovir and pentoxifylline mouth rinses should not be used in an established oral mucositis. At the beginning, mucosal pain could be treated with non-
steroidal anti-inflammatory drugs (NSAIDs) like ibuprofen, and when pain becomes severe, opiates, like morphine, may be used orally (preferred if patients can swallow) or systemically [23]. Topical analgesics (i.e. viscous lidocaine) could be used before eating in order to counteract dysphagia and achieve better pain relief [8,23]. Another suitable option is transdermal fentanyl [23].
Despite the fact that an effective treatment to prevent mucositis in 100% of cases does not exist, its severity can be prevented. During the initial phase of mucositis, there are increased levels of proinflammatory products and NSAIDs administration may help inhibiting the development of severe mucositis. Antibiotics such as mouth rinse (chlorhexidine) and the use of barrier measures are very important for the purpose of minimizing mucosal weakening and preventing epithelium loss. Case series support the benefit of local and systemic corticosteroid therapy to treat mammalian target of rapamycin (mTOR) inhibitor associated stomatitis [23,25-27]. There is strong evidence regarding the paramount importance of good oral hygiene and correct oral health care before the beginning of CT, since the risk of mucositis appearance is reduced [11].
- Oral Cryotherapy is considered a cost effective and proven beneficial method to attenuate CT side effects such as mucositis [23]. Cryotherapy can produce major vasoconstriction, thus restricting the appearance of toxic products in oral mucosa cells. Cryotherapy may prevent mucositis development in up to 50% of cases [8]. It should start 5 minutes before CT infusion and should be kept during all CT cycles. It is especially effective against methotrexate, melphalan, edatrexate, epirubicin and 5-FU [29].
- Low energy laser therapy may heal mucosal membranes, and relieve symptoms thanks to laser’s action over myofibroblastic activity which helps epithelial restitution, thus, improving oral function [29,30]. Helium and neon lasers may produce analgesia [10,31-33]. Although there are studies suggesting that laser therapy may reduce the severity of oral mucositis and pain intensity, and improve the ability to swallow, results are somewhat controversial [3,11,16].
Conclusions
The majority of analysed studies do not exclusively focus on breast cancer populations, mixing up different kinds of cancer and different populations (i.e menopausal or premenopausal women), neither specifying rates of discontinuation of treatment, or the impact on quality of life specifically attributed to mucositis. In the future, these aspects should be taken into account to reach accurate conclusions. Moreover, in many studies a major limitation is the low number of patients recruited for analysis.
The best therapeutic option for treating oral mucositis is still unknown and there is still controversy over which treatment can heal oral mucositis. More studies about oral mucositis focusing on breast cancer are needed since there is no established treatment for the prevention or management of stomatitis. For this reason, it is essential to consider risk factors associated with its appearance and to pay special attention to this entity, considering that it can limit quality of life, and even the survival of these patients.