Introduction
Multiple factors like pelvic surgery, postmenopausal stage, multiple vaginal deliveries, increased body mass index (BMI), connective tissue diseases, and smoking have been implicated in the pathogenesis of pelvic organ descent [1]. Physical examination and imaging are important for the evaluation, staging, and management of pelvic organ prolapse (POP).
Abnormal symptoms and prolapse are caused by connective tissue laxity of the vagina or its suspensory ligaments [2]. Organs are suspended by ligaments. Pelvic muscles stretch the organs against the ligaments to give them shape and support. By a sequence of coordinated contraction and relaxation, the organs are closed (continence) or are opened out actively (emptying). Therefore, the laxity at ligamentous insertion points may cause not only prolapse, but also symptoms of incontinence and abnormal emptying [3]. The anatomical definition of the sign of POP is the descent of one or more of the following: the anterior vaginal wall, posterior vaginal wall, the uterus (cervix) or the apex of the vagina (vaginal vault or cuff scar after hysterectomy) [4].
Stress incontinence occurs when the lax pubourethral ligament fails to anchor the urethra and weakens the force of the pubococcygeus muscle contraction so levator plate and longitudinal muscle of the anus pull open the urethra during effort [2]. The horse-shoe muscle of the urethra and pubococygeus muscle insert into the connective tissue. As the connective tissue of the vagina and pubo-urethral ligament atrophies, these muscles lose their contractile strength. The diminished closure strength and the dilated lumen together lower the intraurethral pressure causing intrinsic sphincter defect. Involuntary urine loss caused by increased abdominal pressure such as laughing, coughing, sneezing is defined as stress urinary incontinence (SUI). Connective tissue laxity in the suspensory ligaments (pubourethral ligaments, uterosacral ligaments and arcus tendineus fascia pelvis) or vaginal membrane may cause bladder instability [2]. Urinary urgency, frequency, nocturia and not being able to hold before reaching the toilet are symptoms of urge urinary incontinence (UUI).
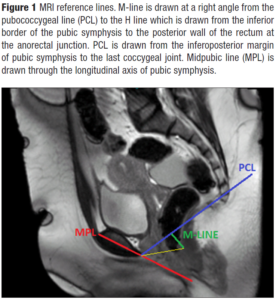
Multiplanar magnetic resonance imaging (MRI) allows the evaluation of muscular and ligamentous structures as well as the endopelvic fascial anatomy of the pelvic compartments with high resolution images. Static MR images visualize pelvic floor anatomy and the defects of the supporting structures, while dynamic MR images visualize pelvic organ mobility and POP [5]. The frequently used reference lines to define POP in dMRI are pubococcygeal line (PCL), mid-pubic line (MPL), and M-line where PCL is the most widely accepted one since it is easily reproducible [6-8].
Dynamic MRI sequences significantly increased the sensitivity of defining and staging MRI based prolapse in symptomatic or asymptomatic patients [8-11]. MRI can detect hidden masked abnormalities, and this may influence the choice of the surgeon’s technique [5]. Th correlation of dynamic MRI findings with symptoms and clinical examinations in POP staging may vary and there are ongoing studies for optimization [12]. The aim of the present study was to evaluate the association of MRI identified POP in women with urinary incontinence complaints but without prolapse findings on physical examination.
Material and methods
This prospective study was approved by the Ethics Committee of our institution which is a tertiary center. Informed written consent was obtained from all subjects. One hundred fifty-eight women with no clinically evident pelvic organ descent detected in gynecological examinations were referred from the Gynecology out-patient clinics to the Radiology Clinic to undergo dynamic pelvic MRI. MRI indications for patient referrals were gynecologic diseases related to the uterus, ovaries, cervix and vagina or menstrual disorders. None of the patients had malignant disease nor were pregnant. Any patient who had any type of pelvic floor reconstructive surgery or hysterectomy was excluded.
Prior to MR examination each patient filled out a questionnaire regarding their medical and obstetric history and was asked if she had any complaint of urinary incontinence (UI) and if so was requested to fill out the urogenital distress inventory (UDI-6) and the Incontinence Impact Questionnaire (IIQ-7). The UDI-6 is a short version of a condition-specific quality of life instrument, the UDI, which was introduced in 1994 [13]. Presently, due to its feasibility, the UDI-6 is much more often used than its longer version. Higher scores in the UDI-6 indicate higher disability. Total score may range from 0 to 100 [13]. The IIQ-7 is a urinary incontinence-specific psychometric questionnaire that assesses the psychosocial impact of UI in women [14]. Total score may range from 0 to 100 [15]. A higher score on both the UDI-6 and IIQ-7 indicate worse symptoms and quality of life [15].
Since the UDI-6 is a condition- specific questionnaire, it has been used as a tool to differentiate symptomatic UI patients from asymptomatic ones. Different thresholds have been proposed [16-18]. The cutoff score of 25 for the UDI-6 has been chosen to distinguish symptomatic women with UI from asymptomatic ones [16].
A simplified pelvic organ prolapse quantification (POPQ) examination was performed by a gynecologist [19]. Women were asked to empty their bladder before the examination and then placed in the dorsal lithotomy position. Once women were positioned for examination, they were instructed to forcefully bear down or perform Valsalva maneuver. If women could not perform the Valsalva maneuver, they were then asked to cough deeply. The four examined areas included the anterior and posterior vaginal walls, the posterior fornix and the cervix [20,21]. For the examination of the anterior and posterior segments, a Heaney retractor was used. For examination of the anterior vaginal segment, the retractor was placed into the vagina and the posterior vaginal wall was retracted to allow for full visualization of the anterior vaginal wall. Regarding the anterior wall, point Ba was identified around 3 cm proximal to the hymenal remnant. The subjects were then instructed to perform the Valsalva maneuver or cough in a forceful fashion and where that Ba point descended in relation to the hymenal remnants was recorded as the ordinal stage of the anterior vaginal wall. The posterior segment was examined in a similar fashion. The point Bp was identified approximately 3 cm proximal to the hymenal remnants. The cervix which was marked as point C was evaluated by placing a speculum in the vagina and directly observing its descent during the Valsalva maneuver or cough to determine its stage in relation to the hymenal remnants. The posterior fornix was designated as point D. Care was taken to make certain that the cervix was not inadvertently supported by the speculum during the examination. The simplified POP-Q staging system was used: Stage 1 prolapse: the given point remains at least 1 cm above the hymenal remnants; Stage 2 prolapse, where the given point descends to an area extending from 1 cm above to 1 cm below the hymenal remnants; Stage 3 prolapse: the given point descends more than 1 cm beyond the plane of the hymen but everted at least 2 cm less than the total vaginal length; and Stage 4: Complete eversion or eversion at least within 2 cm of the total length of the lower genital tract is demonstrated [4,22,23].
Imaging protocol
Patients were requested to void 30 minutes prior to the examination. Intraluminal opasification was not required. To clarify that the individual understood the instructions, the Valsalva maneuver was clearly explained before the procedure. Patients were instructed to increase the intraabdominal pressure by the order ‘strain as if you are defecating’. MRI was performed at rest and during straining in the supine position with slightly flexed legs using a 3T MR scanner (Magnetom Verio, Siemens, Erlangen, Germany). Following standard pelvis MRI, a midline sagittal plane including the pubic symphysis, vaginal apex, rectum, and the coccyx within the field of view was selected for acquisition of a dynamic true fast imaging with steady state free precession sequence. Patients were instructed to strain for 10 seconds. This procedure was repeated twice to obtain the most optimal patient cooperation regarding straining [8]. Acquisition parameters were as follows: TR/TE 4.96 /2.08 ms, 60° flip angle, 254 FOV, 230 x 256 matrix, 5 mm slice thickness.
Image interpretation
The images were evaluated on the workstation by a radiologist with twenty years of experience. Midsagittal images at rest and on strain were used for evaluation. M-line, PCL, and MPL were used to detect and grade prolapse in three pelvic compartments (Figure 1).
M-line, corresponding to the vertical descent of the levator hiatus, was drawn at a right angle from the PCL to the H line, which was drawn from the inferior border of the pubic symphysis to the posterior wall of the rectum at the anorectal junction [8]. Pelvic floor descent was defined when M-line exceeded 2 cm.
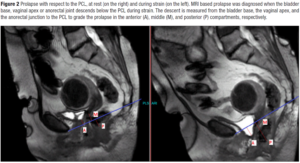
PCL was drawn from the infero-posterior margin of pubic symphysis to the last coccygeal joint. The perpendicular distance from PCL to the bladder base, the vaginal apex, and the anorectal junction graded the degree of the descent in the anterior, middle, and posterior compartments, respectively [8] (Figure 2). MRI based prolapse was diagnosed when the bladder base, vaginal apex or anorectal joint descended below the PCL during strain. POP was graded as negative, descent to less than 1 cm; mild, 1-2 cm; moderate, 2-4 cm; severe, more than 4 cm inferior to PCL [8,10].
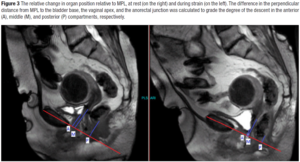
MPL was drawn through the longitudinal axis of the pubic symphysis and the perpendicular distance from MPL to the bladder base, the vaginal apex, and the anorectal junction was measured (Figure 3). The relative change in the anatomic position was measured relative to MPL between rest and strain by substracting the two measurements from each other [8]. MRI based prolapse was graded as negative, no decent; mild, increased descent with strain of 0.5-2 cm; moderate, increased descent with strain of 2-4 cm; severe, increased descent with strain of more than 4 cm relative to MPL [8,24-26].
Primary outcome measures were the presence of MRI based prolapse in relation to UI complaints. The secondary outcomes were the associations between some of the risk factors and UI complaints.
Statistical analysis
Statistical analysis was performed with StataSE 10.0 (Statacorp, Texas, USA). Demographic characteristics were expressed by using descriptive statistics. The Student’s T-test was used to compared continuous variables and the χ2 test and Fisher’s exact test were used for categorical variables. Results are expressed as mean and 95% confidence intervals. Correlations between variables were calculated by the Spearman test. Multivariable logistic regression modeling was used to compute the odds ratios (ORs) of variables predictive of UI complaint. A value of p of <0.05 was considered as statistically significant.
Results
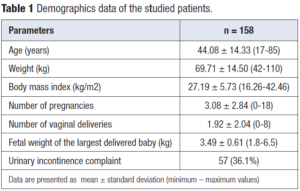
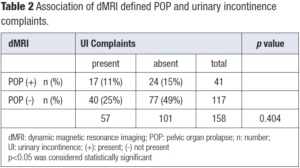
Patient demographics are given in Table 1. The association of dMRI defined POP findings and UI complaints are summarized in Table 2. Fifty-seven of the patients had UI complaint and seventeen of those had dMRI detected POP (11% of the study group). One hundred and one women had no UI complaint and twenty-four of those had dMRI detected POP (15% of the study group). One fourth of women enrolled in the study without visible prolapse on physical examination turned out to have dMRI detected mild vaginal apex prolapse which was weakly correlated with UI. Forty of the women with UI complaint and seventy-seven of those without UI complaint had no dMRI detected POP (25% and 49% of the study group, respectively).
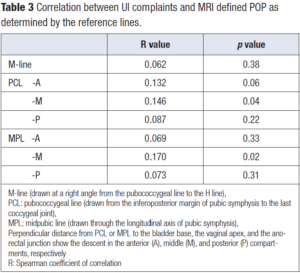
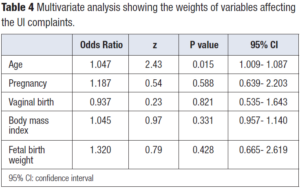
The correlation between UI complaints and various MRI parameters is presented in Table 3. There was weak positive correlation between UI complaint and POP detected at the middle compartment when PCL and MPL were used as reference lines (correlation coefficients 0.146 and 0.170, respectively). UI complaint was not correlated with the number of pregnancies, vaginal births, maternal BMI or birth weight of the infant. Patient age was found to be the only variable correlated with UI complaint (Table 4).
Discussion
The present study found that dMRI defined POP, not confirmed by physical examination, was not correlated with UI complaints. While cut-off values for UDI-6 have been proposed for discriminating those women with UI complaints and those without, there is overlapping of scores between the continent and incontinent women. How women perceive UI and answer the questions of the UDI-6 can be influenced by many factors. The answers are subjective and might not reflect the real clinical state of UI. The present study showed that not all dMRI defined POP situations lead to UI.
Forty-nine percent of the patients enrolled in the study had no UI complaint and no prolapse was found upon dMRI. Fifteen percent of the study population had mild prolapse findings on dMRI even if they had no complaints. These patients can be named silent POP patients since they have no visible POP on examination. Previous studies dealing with asymptomatic patients have shown that POP can be diagnosed in the absence of levator plate abnormalities or muscle weaknesses but in the presence of endopelvic fascial defects or pure connective tissue disorders. These asymptomatic patients may present problems later on, and therefore should be followed-up [12,27].
Twenty five percent of the study group with UI complaint had no prolapse findings on dMRI and no visible POP on physical examinations. In these cases, the reason could have been related to urge UI or overactive bladder. Since cystometry was not performed to any of the patients, the cause could not be determined.
Eleven percent of patients enrolled in the study with UI complaints had positive prolapse findings on dMRI. The apex of the vagina is marked by PCL-M and MPL-M, with the supporting connective tissue structures such as both uterosacral ligaments and rectovaginal fascia. In our study, a weak positive association between UI complaint and POP in the middle compartment was identified by dMRI when PCL and MPL were used as reference. However, this weak association could have been related to the fact that the dMRI defined POP was not visible during pelvic examination.
The apex of the bladder is marked by PCL-A and MPL-A, with the supporting connective tissue structures such as the pubocervical fascia and the arcus tendineus fascia pelvis. The descent with respect to the reference lines with strain was not associated with UI. The possible explanation could be that the women in our study had no physically detected anterior vaginal wall prolapse.
PCL and MPL, each have shown in different studies good detection for defects at the anterior and middle compartments [7,17]. In some studies, which have examined symptomatic patients, MPL detected prolapse better than the other lines; whereas in other studies PCL was found to be superior [25,26]. Our patients were asymptomatic in terms of POP. In dMRI defined POP studies, posterior vaginal wall prolapses were found to be more frequent than the anterior vaginal wall prolapses where the higher mobility of the posterior vagina was addressed as the cause [8]. Our results are in accordance with the published data. However, the defined by dMRI did not result in clinical POP symptoms yet.
MRI parameters defining POP were not correlated with any of the risk factors for POP like parity, vaginal delivery, fetal birth weight or BMI as we would have expected, since our study group consisted of patients with no clinical POP complaints. Nevertheless, patient age was the only variable that showed correlation, which was not unexpected.
There are some limitations of the present study. First of all, no women with UI complaints had urodynamic evaluation. Secondly, the incontinence type was not categorized. Patients with UI were treated in our study as one group (symptomatic) without further dividing them into SUI, MUI and OAB subgroups. Thirdly, the cut-off value used for the present study was adapted from a study performed in another country. The way patients complain about their symptoms might have socio-cultural, regional, religious and educational differences.
In conclusion, there was weak positive correlation between UI complaint and POP detected at the middle compartment when PCL and MPL were used as reference lines. The likelihood of dMRI detected POP was is similar in women with UI complaints when compared to those without UI complaints. Although dMRI shows subtle anatomical abnormalities in connective tissue with high resolution [28-30], those anomalies will not lead to UI unless physically diagnosed POP is present. The interpretation of questionnaires aimed at determining the severity of UI and its impact on individual quality of life should rely on population-based studies. Subjective evaluating tools, such as the UDI-6, should be used together with objective tools such as dMRI for the diagnosis, treatment and follow-up of patients with symptoms of UI and/or POP.
Compliance with ethical Standards
This study was approved by our Institutional Ethics Committee which is a tertiary center. All subjects gave informed consent to participate in the study.