Introduction
Ovarian benign tumours are a widespread disease of the women’s reproductive system. There is no consensus on the definition of giant ovarian tumours (GOT), but most authors use the term to refer to masses with a maximum diameter of 15-20 centimetres or cysts reaching above the umbilicus [1]. In recent years, these giant neoformations have become a rare finding in high-income countries because of the increasing development of public health systems and the advantages of medical diagnostics and screening programs.
Ovarian cysts are common benign gynaecological conditions that affect about 4% of premenopausal women and are generally self-resolving [2]. Ovarian cysts in postmenopausal women are also common, with 15-18% incidence of simple ovarian cysts [3,4] and 21% of abnormal ovarian morphology [5]. About 69% of ovarian unilocular cysts are benign, commonly asymptomatic, and resolve spontaneously, especially when they are small and with no additional complications. However, a small portion of these growths may be complicated by pain, haemorrhage, cyst rupture and adnexal torsion, which may necessitate urgent hospitalisation and surgical intervention to avoid further clinical complications. GOTs are commonly found in obese patients who experience a cohort of unspecific symptoms for an extended time, frequently without asking for medical assistance. The typical symptomatology includes progressive abdominal distension accompanied by nonspecific diffuse abdominal pain and signs and symptoms derived from the compression produced by the mass on the nearby organs (“ab extrinseco” symptomatology) such as bowel dysfunction, dyspepsia and urinary tract disorders [6]. Surgical intervention for a GOT is mandatory, especially if the cyst is rapidly growing and is causing severe cardiopulmonary distress. However, in selected cases, when the mass does not present a malignant appearance and does not cause severe multi-organ dysfunctions, in the absence of patient compliance to the surgery, it is possible to delay the surgical approach and to set a strict follow-up with imaging and oncomarkers. Nevertheless, there is no real consensus on the most appropriate protocol to follow in the management of such complex cases.
The histopathological exam is essential for counselling women towards the most appropriate follow up or to set the necessary consequent medical therapy [7,8]. The surgical excision of the mass requires a multidisciplinary team and a high-specialized hospital care. The undoubted and well-documented advantages of laparoscopy on the duration of surgery, complication rate and total days of hospitalisation [9] should be weighed against the technical difficulties when accessing an abdominal cavity fully occupied by a giant abdominal mass. We report a rare case of a GOT with a major diameter of 40 cm and a weight of 30 kg found in a 58-year-old woman with multiple comorbidities, diagnosed and managed in our university hospital.
Methods
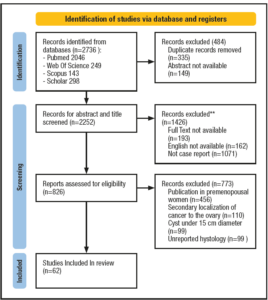
We retrieved the data for the case report from our hospital’s registry. Our study received a pre-acceptance by PROSPERO (CRD42023440145) [10]. The research and data extraction for studies eligible for analysis for this review were performed according to the PRISMA guidelines [11]. We identified 2,736 papers eligible for our review, which was comprehensively scoped via the medical database of PubMed, Web Of Science, Scopus and Google Scholar. We focused on the following keywords in different combinations: giant ovarian cyst, massive ovarian tumour, giant ovarian cancer, management, and risk factors. To include all the spectrums of this rare pathology, we set a wide time frame for the publication dates that covered articles published from 1982 to 2023 and a limit to case reports only. We screened the selected reference list of articles to find additional eligible publications. Reviewers #1 and #2 worked independently to screen all the titles and abstracts. Disagreements were settled through consensus with reviewer #3. The inclusion criteria were (1) full-text articles, (2) papers written only in English, and (3) ovarian cysts more prominent than 15 cm in menopausal women who underwent surgical and medical management. We used the cut-off of 15 cm in line with the current literature, where most authors use the term “giant” ovarian masses referring to masses of 15-20 cm of diameter or reaching above the umbilicus. Reasons for exclusion were publications in premenopausal women, secondary localisation of cancer to the ovary (n=110), cyst under 15 cm diameter (n=99), unreported histology and articles not pertinent to the topic. Reviewers #4 and #5 worked on the data extraction, creating a standardised extraction table including information about the PubMed unique IDentifier, year of publication, author, age and body mass index (BMI) at diagnosis, size and weight of the tumour, presence of ascites and acute abdomen at diagnosis, assessment of oncomarkers, surgical details like side, performed surgery, type of surgery and post-surgical data about histopathological findings and, in case of malignancy, final tumour staging. We also collected data regarding the follow-up or use of adjuvant/neoadjuvant chemotherapy. Discrepancies were resolved through discussion with reviewers #1 and #2. Reviewers #4 and #5 evaluated the quality of studies by independent screening using a modified ROB 2 scale [12], evaluating three items and a final overall risk of bias. The assessed items were: deviation from intended intervention bias, missing outcome data bias and measurement bias. Each item was evaluated on a three-point scale with low, some concerns, high and no information. We excluded randomization bias and selection bias because the included studies were all case reports.
Results
Case presentation
The patient was a 58-year-old Caucasian woman who was transferred to the gynaecological surgery unit of the University of Bari “Aldo Moro” clinic for the completion of the diagnostic workup and surgical treatment of a 45 cm abdominal mass.
Regarding the patient’s obstetrical anamnesis, she had one vaginal delivery of one living child and a spontaneous miscarriage (9 weeks). The patient had her menarche at 14, and she went through physiologic menopause at 48 years of age. The patient’s only postmenopausal gynaecological complaint was a mild urogenital syndrome.
The woman’s general medical history included multiple morbidity such as anaemia, type III obesity (BMI 55.9 kg/m2), chronic obstructive pulmonary disease, asthma and hypertension. She had no personal history of major surgery and no family history of malignancy or hereditary diseases. She reported a gradual increase in the volume of her abdomen with concomitant ongoing symptoms of mild constipation, abdominal pain, urinary incontinence and dyspepsia over the previous 3-4 years. Upon hospital admission, she complained of tension-type abdominal pain. Upon the clinical objective evaluation, she appeared particularly pale and dyspneic. Her weight was 138 kg with a height of 157 cm; other vital parameters were within normality range (body temperature of 36.8 °C and a blood pressure of 120/70 mmHg).
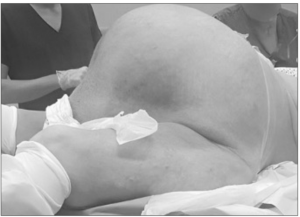
At inspection, the abdomen was largely distended, (abdominal girt of 190 cm) showing collateral venous trajectories on the skin. The palpation of the abdomen revealed an enormous abdominopelvic mass with a dull note on percussion, but with normal peristalsis (Figure 1). The assessment of the lower limbs highlighted bimalleolar oedema, loss of muscle volume and atrophic skin changes.
Transvaginal ultrasound was not executed due to the low compliance of the patient.
The transabdominal ultrasound revealed the presence of a 40 cm complex unilocular mass that occupied the whole abdominal cavity, suspicious for serous cystadenoma.
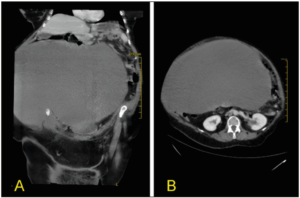
Computed tomography (CT) scan highlighted the presence of a complex cystic intraperitoneal lesion of 41 cm (craniocaudal) x 27 cm (anteroposterior) x 40 cm (transverse) with homogeneous iso-hypo dense (Hunsfield Hunit (HU) 25-40) content and regular margins, with no evidence of septation and pathologic impregnation with the contrast agent (Figure 2). The cyst had a significant mass effect on the nearby organs but without signs of neoplastic invasion. The abdominal organs appeared normal except for a cholesteric stone in the gallbladder and a small cortical cyst in the right kidney. There was no compression of the urinary tract and there was no free fluid in the abdomen. The radiologist's first hypothesis regarding the nature of the mass was that of a giant serous ovarian cystadenoma.
Serum tumour markers however were over range: cancer antigen 125 (CA-125) 2,964 U/mL, cancer antigen 19.9 (CA-19.9) 166 U/mL and the human epididymis protein 4 (HE-4) 195 pmol/L with a slight increase of the carcinoembryonic antigen (CEA) 5.3 ng/mL and normal alpha-fetoprotein (AFP). Risk of Ovarian Malignancy Algorithm (ROMA) score [13] was calculated and showed a 93.3% risk of finding an epithelial ovarian cancer. A routine blood test showed the following results: Hemoglobin: 13.3 g/dL, white blood cell count 10.98 x 103/uL, platelets 283 x 103 /uL, in addition to normal renal function (EGFR 101 ml/min, creatinine 0.6 mg/mL), and electrolytes. Liver enzymes and coagulation were also normal. Thyroid functional tests were regular. There was a slight hypoalbuminemia (3.2 g/dL) and a slight increase of C-reactive protein (9.3 mg/L). The urine test and virological exams were negative. The routine electrocardiogram showed sinus bradycardia at 54 beats per min and the chest X ray was negative for significant abnormalities.
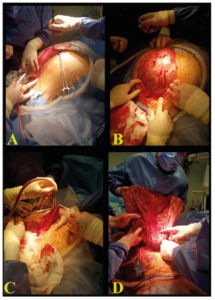
The surgery was performed on a bariatric operative table, with the patient under general anaesthesia, after preparing a sterile operative field and inserting a Foley catheter in the urinary bladder. We performed a midline longitudinal laparotomy incision from the lower abdomen up to the xiphoid process (Figure 3 A). After opening the abdominal walls, we encountered the presence of a gigantic neoformation with smooth regular walls (Figure 3 B). A small 2 cm incision on of the top of the cyst was performed to allow the insertion of the surgical vacuum: 28 centilitres of dense meta-haemorrhagic fluid from the inside of the cyst were aspirated (Figure 3 C).
The gross appearance of the internal wall of the cyst was not suggestive of malignancy, with its regular inner wall linings and small papillary intra-cystic projections without evidence of extracapsular growth. The depletion of the cyst’s fluid content permitted the exploration of the entire abdominal cavity. The left ovary appeared entirely transformed by the cyst. The cyst’s wall was adherent to the abdominal wall, the peritoneum, the omentum, the uterus, the bladder and the intestine. The uterus and contralateral adnexa appeared macroscopically normal. Adhesiolysis was performed, followed by total abdominal hysterectomy, bilateral salpingo-oophorectomy, omentectomy and appendicectomy. The examination of the whole small intestine, from the Treitz to the ileocecal valve, revealed a tubular structure suspicious of a diverticulum of Meckel; therefore, an intraoperative general surgery consultation was requested. The tubular structure was removed by tangential resection with Echelon Elex gold charge, and a 2.5 cm area of longitudinal desperitonizations at the level of the distal sigma (negative for colic perforation at the hydropneumatic test) was reinforced with serum-serum points in PDS 2/0. Normal haemostasis was achieved. Two surgical drainage tubes were left with one in the Douglas and one in the paracolic right fossa. The fascia was closed by an interrupted size two polyglactin 910 suture; the subcutaneous tissue was closed with an interrupted size one polyglactin 910 suture. The skin incision was sutured by a sterile single-use skin stapler with 45 stainless steel staples. Remarkably, the enlarged abdomen of the patient now appeared flat at the end of the surgical procedure, indeed the excised cyst measured 40 cm in its long axis (Figure 3 D). The surgery lasted a total of about four hours. The estimated blood loss was 300 cc.
The postoperative recovery was uneventful, with the patient being discharged from the hospital on the 13th day after the procedure only because of the pre-existing comorbidities. The post-operatory therapy included fluids, fasting for five days and an antibiotic therapy consisting of ceftriaxone plus metronidazole full-dose. The thromboembolic prophylaxis included the administration of subcutaneous enoxaparin injections and the use of compression socks on the lower limbs. The patient was left with a nasogastric tube for the first 24 hours after the surgery to drain any gastric secretion. The patient’s mobilisation started on the fifth day with the aid of a physiatrist. There were no symptoms of bowel obstruction, and the patient’s alvus returned to the norm on the second postoperative day and the diuresis was normal. We removed the paracolic drainage on the seventh day and the left abdominal drainage until the ninth day. The transurethral Foley and part of the suture clips were removed on the 12th day, with the remaining ones removed five days after the hospital discharge. There were no significant complications during the immediate 45 days after surgery.
On the histological exam, the ovary was entirely transformed into a discontinuous cystic neoformation containing coagulated blood with a diameter of 40 x 36 x 34 cm. On the internal surface of the cyst, there were multiple intracapsular vegetations. The salpinx was 5 cm in length. The final diagnosis was of borderline serous ovarian tumour with intact capsule and no extracapsular vegetations. The salpinx, uterus, omentum, appendix and peritoneum specimens were neoplasm-free.
The case was discussed with our hospital's multidisciplinary oncological team with the final indication of a six-month follow-up. No genetic counselling was made due to the borderline nature of the neoplasm [14].
Literature review
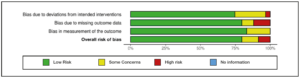
We used IBM® SPSS® to perform the statistics. A total of 62 records were included in our review. After removing duplicate articles and articles with unavailable abstracts, reviewers #1 and #2 screened 2252 papers based on their titles and abstracts. Excluding articles without available full text and in a language different from English and not case reports, 826 records remained. Reviewers excluded 773 articles that did not match the inclusion criteria. The remaining 62 case reports assessed for eligibility were processed by reviewers #3 and #4. We identified 62 case reports. The statistical tools that we used were T test to compare means and chi square test to examine categorical variables. We assessed the risk of bias via ROB2 tools. The results, showing an overall low risk of bias, are presented in Figure 4. The PRISMA 2020 flow diagram of the study selection process is presented in Figure 5.
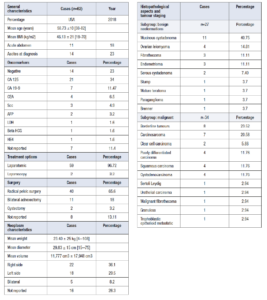
We enlisted 62 cases of postmenopausal GOTs, as summarised in Table 1. The mean age at diagnosis was 58.73 ± 10 years. The youngest woman was 38 [15], and the oldest was 82 [16]. Patients’ mean BMI was 46.13 ± 21 kg/m2. The patients’ clinical presentation was not specific, with only 18% presenting an exordium with acute abdomen (n=11) and 23% with ascites at diagnostic imaging (n=14).
The oncomarkers were negative in 23% of patients (n=14). CA 125 was positive in 34% of the patients (n=21) and 44.1% (n=15) of the subgroup diagnosed with malignant tumours. CA 19-9 was positive in 11.47% of patients (n=7). CEA (n=4), SCC (n=3) and AFP (n=2) were positive in a small portion of patients. LDH was found positive in one case [17]. CA 15-3 was not found positive in any (n=0). Beta HCG was positive in one case of metastatic epithelioid trophoblastic tumour [18]. HE4 was found positive in one patient [19].
We highlighted that the laparotomic approach was chosen in 96.72% (n=59) of cases, with only two reported laparoscopy cases in benign and small mass cases [15,20]. No authors used robotic surgery (n=0). The treatment of choice was, in 65.6% of cases, radical pelvic surgery (n=40) with hysterectomy and bilateral salpingo-oophorectomy followed in most cases by omentectomy and appendectomy in the suspicion of mucinous tumour. An 18% of surgeons performed bilateral adnexectomy (n=11). Only two cases of cystectomy were reported [15,21]. Regarding the side location, we found 36.1% (n=22) right-sided cysts, 29.5% (n=18) left-sided, and 8.2 % (n=5) bilateral. The tumour’s mean weight was 23.40 ± 25 kg. The heaviest was 108 kg [22]. The mean diameter of the tumour was 29.83 ± 15 cm. The largest was 75 cm long [22]. We used the ellipsoid formula (x for y for z for a constant of 0.52) to approximate neoplasm volume. Using this estimation, we calculated the mean volume of the neoplasms of 11,777 cm3 ± 17,948 cm3.
In our review, the histopathological results of GOTs found a prevalence of 55.73% of malignant (n=34) and 44.26% of benign (n=27) neoformations. Benign histopathology was represented in 40.75% of cases by mucinous cystadenomas (n=11) and ovarian leiomyomas in 14.81% of patients (n=4). Mildly common tumours were fibrothecoma (n=3) and endometrioma (n=3), assessing, respectively, the 11.11% of benign GOTs. More rare tumours are serous cystadenomas, 7.40% of cases (n=2) and isolated cases of STUMP (smooth muscle tumour of uncertain malignant potential), ovarian paraganglioma, benign Brenner and teratoma, assessing for about 14.8 % of cases. Among malignant GOTs, we found a high percentage of borderline tumours, 23.52 % (n=8), with a prevalence of mucinous, serous and Brenner histology (50%, 25% and 25% of cases, respectively). Carcinosarcoma and sarcoma had a higher prevalence, among 20.58% (n= 7). We found 2 cases of clear cell carcinomas (5.88%) [23,24]. A 31.14 % of patients had malignant and poor prognosis histopathology: carcinosarcoma (n=7), poorly differentiated carcinoma (n=4), squamous carcinoma (n=4) and cystadenocarcinoma (n=3). We reported 7 cases of isolated tumours with rare histology: Sertoli Leydig [25], urothelial carcinoma [26], malignant fibrothecoma [27], granulosa tumour [28], trophoblastic epithelioid metastatic tumour [18], Brenner [29] and ovarian paraganglioma [30].
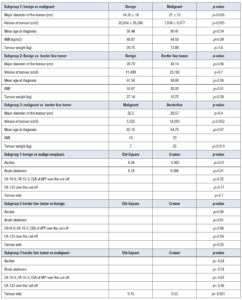
Comparing malignant and benign neoplasms, we found a statistically significant difference between the major diameter of the tumour, that was 34.35 ±18 cm in benign and 27 ± 12 cm in malign tumours (p<0.035, Coehn’s d= 14.56 CI -0.41 – 1.15) and volume of tumour estimated with ellipsoid formula that was 20,634 ± 26,386 cm3 in benign and 7,638 ± 9,577 cm3 in malignant tumours (p<0.025, Coehn’s d=16759 CI -0.11 – 1.72). These results demonstrate that the evidence of a large tumour is more frequently correlated to a benign origin. We also found that there was no statistical difference between mean age at diagnosis (p=0.14), BMI (p=0.39) and tumour weight (p= 1.6) between benign and malignant tumours. We performed a statistical analysis to determine common characteristics that clinicians can use to assess the probability of malignancy. We found a statistically significant correlation between ascites and malignancy (p<0.01, Chi-Square 8.04 Cramer 0.363) with a 57.,1% sensitivity and 33.3% sensitivity. There was also a statistically significant correlation between acute abdomen and malignancy (p<0.01 Chi Square 8.18 Cramer 0.366) with a sensitivity of 54.5% and a specificity of 33.3%. We did not find a statistically significant correlation between oncomarker positivity (p=0.22) and malignancy. There was a high sensitivity (71.4%) and sensitivity (50%) for CA 125 related to malignancy, but it was not statistically significant (p=0.17). There was no statistical significance for the tumour side (p=0.10), but we can assume that 72.7% of malignant tumours can be found on the right annexe.
Subgroup analysis was performed by differentiating borderline ovarian tumours vs. benign tumours. No statistically significant difference was found between Borderline and benign tumours in terms of volume (p= 0.7), major diameter (p=0.96), weight (p=0.39), age (p=0.56) and BMI (p=0.51). No statistical correlation was found between borderline histology and ascites (p=0.90), acute abdomen (p=0.81) oncomarkers (p=0.96) or surgical side (p=0.33).
There was a statistically significant difference in the weight of the tumour (p<0.013, Cohen 7.14 CI 95% -4.52 – 0.51) and volume (p=0.052). The mean weight of malignant tumours was 7 ± 3.93 kg, while the main borderline weight was 25.33 ± 11 kg. The mean volume of the malignant tumour was 5,522 ± 6,895 cm3 versus the mean borderline volume, which was 14,203 ± 12,905 cm3. We also found a statistically significant difference in the tumour side (p<0.021 Chi-square 9.74, Cramer 0.53), where borderline tumours were more uncommon on the right side than malignant neoplasms. There was no statistically significant difference between borderline and malignant tumours in terms of comparing diameter (p=0.4), ascites (p=0.64), acute abdomen on the onset (p=0.54) and oncomarker expression (p=0.63).
Finally, we performed a one-way ANOVA to compare the three groups benign vs borderline vs malignant. There was no statistically significant difference between borderline and benign tumours in volume (p= 0.51), major diameter (p=0.08), weight (p=0.24), age (p=0.06) and BMI (p=0.26). All the extensive statistics are in presented in Table 2.
Discussion
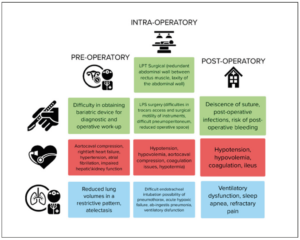
The clinical signs and symptoms of GOTs are usually non-specific. According to our findings, only 18% of patients experience acute abdomen upon hospitalisation; patients usually experience a slow onset of non-specific symptoms, recurrent abdominopelvic pain and a concomitant increase in abdominal circumference with a feeling of abdominal heaviness [31]. Our patient experienced non-specific symptoms for 3-4 years. She was severely obese (BMI 59.99 kg/m2), a condition that masked the distention of the abdomen caused by the mass. She confused her symptoms with other aetiologies and thus, did not seek medical attention. Giant abdominal masses are frequently associated with constipation [32–34], cardiovascular, pulmonary and urological signs and symptoms. Common gynaecological signs are menstrual irregularities [35] and dyspareunia [36]. Our patient was affected by hypertension, asthma and chronic obstructive pulmonary disease, clinical conditions which complicated diagnostic workup, surgery and post-operative management. In Figure 6) we summarised the common potential problems that must be considered in the pre-operative, intraoperative and postoperative management of GOTs.
Transvaginal ultrasonography (TV-US) is the imaging tool of choice for abdomen/ pelvic neoformations, but presents technical difficulties when performed on extremely obese patients. Every adnexal mass should be studied with ultrasound for a correct differential diagnosis between benign/borderline/malignant disease; suspicious signs of malignancy include papillary projections, septums or solid masses on the cyst’s wall.
The IOTA (International Ovarian Tumor Analysis) simple rules [37,38] and the Risk of Malignancy Index (RMI) [39] are simple and effective score used for triaging adnexal masses in women. The execution of the TV-US was complex in our patient because of the technical difficulties and the poor compliance. However, the clinical features were indicative of a tumour of a benign/borderline nature. ROMA score was calculated for 93% risk of epithelial ovarian cancer. Considering the general principle of “as low as reasonably achievable” [40], the diagnostic workup reserves MRI\CT scans for selected patients like ours. This evidence, in association with clinical evaluation and determination of ROMA score, oncomarkers and full laboratory assessment, should be useful in managing GOTs. A CT scan with contrast was executed in our patient to assess the origin of acute abdominal pain and to differentiate the source of the abdominal mass. CT can distinguish between soft and hard tissues and by using contrast agents one can obtain information on the vascularisation of abdominal masses. A MRI is preferred because of the absence of radiation and better resolution of soft tissues, but it is not executable in the urgent setting due to time and compliance issues. Despite the valuable information obtained from radiologic diagnostics and hence its contribution to the diagnosis process, the certain diagnosis is reached only after surgical exploration and by the histologic exam. In elective settings, assessment of serum tumour markers (CA-125, CEA, AFP, beta HCG, CA 19-9, CA 15-3) is mandatory, especially considering its relevance for serial evaluation during the follow-up [41,42]. The integration of CA-125 with HE4, for example, helps to assess the ROMA score. Our study found no statistically significant correlation between oncomarker expression and the risk of malignancy; however, oncomarker rise after the surgery may evidence the possible presence of recurrence, metastases or residual disease.
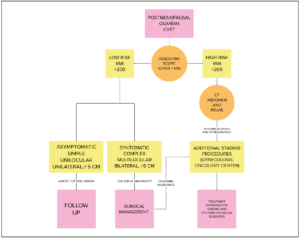
Small ovarian cysts may be aspirated, but there is a high risk of recurrence and some doubt on the possible metastatic diffusion in case of malignancy. Surgical excision is the most suitable treatment for most GOTs. There is an ongoing debate on the surgical strategy of excision to be chosen between laparoscopic or laparotomic techniques. The laparoscopic approach is the gold standard for most benign ovarian cysts to be selected as an alternative to laparotomy (LPT) in elderly patients [43]. A lower postoperative complication rate (7% with LPS vs. 37% with LPT) and fewer days of hospitalisation (5 with LPS vs. 11 with LPT) have been reported. However, the laparoscopic approach for a big abdominal mass is challenging and may be of high risk because of the chance of spillage and dissemination of the cyst’s content into the abdominal cavity. The classic laparotomic approach minimises the risk of intraperitoneal dissemination caused by cell overflow and ensures oncologic radicality while treating the disease. We preferred a laparotomic approach with midline incision because of our patient's high rate of comorbidities. The excision of the cyst in toto with an integral capsule should be performed when technically possible to ensure oncological radicality. Despite this, most surgeons prefer to aspirate the cyst to reduce its volume and allow less complicated dissection, especially in case of resistant adherences, like in the case we report. The cyst drainage was performed with a rigid high-pressure suction cannula. In Figure 7, we propose an integrated diagnostic/therapeutic workup algorithm for cystic lesions in postmenopausal women [37].
Main limitations of the present study
Our study assessed the retriable literature from the most common medical science websites. However, this search has reported bias because bad outcomes are usually not published. We also avoided literature in languages other than English and articles without full-text availability. In addition, we discovered a significant heterogeneity among the case reports and a lack of literature on prospective or retrospective studies related to “giant” ovarian neoplasia. These limitations are probably related to the rarity of the disease.
Conclusions
Our review, the first literature we know and are aware of, aimed to assess the correct diagnostic workup and the prognostic factors that should be considered when dealing with postmenopausal women with these giant neoplasms. A giant abdominal cyst stresses modern hospitals' medical resources with advanced technologies and up-to-date protocols. A multidisciplinary team is mandatory to manage the multi-morbidities that are commonly frequent in these patients: psychosocial, nutritional, surgical, anesthesiological, gynaecological, radiological and more competencies are necessary to successfully manage these patients. The standard protocols and guidelines are sometimes unfit to manage the clinical needs emerging from GOTs. Typically, these patients are managed satisfactorily in a high-specialisation centre. However, in lower-income settings the situation may sometimes have to be dealt in smaller clinical centers. Almost one-third of GOTs are from benign and borderline histology; however, today, radical pelvic surgery is the surgery of choice to assess oncological radicality and to ameliorate the gastrointestinal, urinary and pulmonary distresses caused by the mass. Laparoscopy should be done by experienced, highly trained surgeons in high-specialisation settings. The use of robotic surgery is to be used in selected cases. We didn’t find a statistical significance of mean age at diagnosis, BMI, and tumour weight to determine benign vs. malignant histology but those findings could be limited by the small sample size and heterogeneity of patients and size of abdominal masses. On the other hand, these factors should be prognostic for the patient’s general health. Surgery safety in patients affected by such pathologies is reduced by severe comorbidity, complicating the anaesthesiologic and post-operative recovery. Our results confirm the need for a multidisciplinary, high-specialisation gynaecological oncology team that should diagnose and treat every patient with these complex pathologies in a tailored approach that should cure the patient in accordance to her needs and expectations.
Compliance with ethical standards
The authors thank the patient for permission to present this case report in order to sensitise practitioners. The ethical committee of the hospital approved the publication.
Authors’ contributions
Giuseppe Muzzupapa (GM) (Reviewer #3), Luca M Schonauer (LMS) (Reviewer #4) and Nico Picardi (NP) operated on the patient and followed her up postoperatively.
Marco Cerbone (MC) (Reviewer #1) and NP (Reviewer #2) scoped the literature. MC prepared the images and cured statistics. Iris Cara (IC) cured the English formatting and language editing. MC, NP, IC and Gianluca R. Damiani (GRD) prepared the manuscript draft. MC, IC, NP, GM, LMS and GRD reviewed the original draft. MC, IC and LMS edited the final manuscript. Edoardo di Naro, GM, and Ettore Cicinelli directed the work. All authors read and approved the final manuscript.
Funding
None.
Study registration
The study was pre-registered in PROSPERO.
Ethics approval
Not applicable.
Consent for publication
Written informed consent was obtained from the patient to publish this case report and any related images.
Competing interests
The authors declare having no competing interests.