Introduction
Menopause is the physiological phase of women in which functional failure of the ovaries occurs, causing an abrupt drop in estrogen levels and, as a consequence, not only the end of the reproductive period, but also several morphofunctional changes in women [1]. In the menopausal phase, estrogen deficiency alters the normal bone formation cycle, increasing resorption activity by osteoclastic activation at a faster rate than osteoblastic activity, causing bone weakness [2].
According to research presented by Wong et al. [3] the rate of bone mineral density (BMD) loss in the late perimenopause may be higher than in the early years of menopause. In this way, they considered it important to research a large number of women to analyze the factors interfering in this process, including whether there is an influence of vasomotor changes in these BMD losses related to the physiological life cycle of women [3].
Bone tissue remodeling occurs mainly in the first two decades of life, reaching peak BMD around age 30. In general terms, the greater density obtained, the greater the concentration of bone that will have to be lost over the following years and thus, at least physiologically speaking, osteopenia or osteoporosis may later develop [4].
Osteoporosis affects about 30% of postmenopausal women and 50 to 80% of men with factors that contribute to osteoporosis. Osteoporosis in postmenopausal women and in men in the absence of disease is classified as primary osteoporosis. When it occurs in the presence of a disease or use of medications or special situations, it is called secondary osteoporosis [5].
The main consequence caused by osteoporosis are fractures. Fractures due to osteoporosis occur at an increasing incidence at all ages, but women have twice the risk of fractures than men, making postmenopausal osteoporosis one of the most common and important types of primary osteoporosis [6]. The aim of the present study was to assess the effect of menopause on BMD considering the time of onset of this event.
Material and methods
Sample
The descriptive analysis was carried out through the sample data obtained from the database of the research group of the Densitometry Laboratory of the Federal University of Technology - Paraná- Brazil. The sample consisted of 153 postmenopausal women divided into two groups: 88 subjects with menopause onset before age 50 years (BA group; including age 50) and 65 subjects with menopause onset after 50 years (AA group). This research is in accordance with the norms established in the Declaration of Helsinki and in the resolution 466/2012 of the National Health Council regarding research involving humans.
Inclusion and exclusion criteria
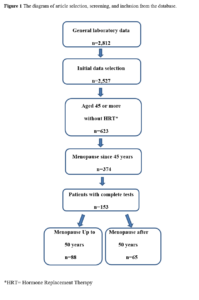
All participants included in the study were postmenopausal women as defined by the modified Reproductive Aging Workshop (STRAW) staging system. Incomplete data of participants were excluded for analysis (Figure 1).
Measurements
All personal and anthropometric data were obtained by direct selection from the laboratory's database. Body composition assessment was performed using Dual Energy X-Ray Absorptiometry (DXA) (Hologic # Discovery, Mississauga, Ontario, Canada). The equipment's own database provided the parameters of total body composition and bone quality. Android/gynoid (A/G) relationship was also determined.
Statistical analysis
Data tabulation was performed using Microsoft Excel version 2015, and data analysis was performed using SPSS software version 21.0. The analysis of the normality and homogeneity of the sample was performed using the Kolmogorov-Smirnov test, which determined the normality of data distribution. Thus, for comparison between groups, the Wilcoxon test was used for two related samples. To verify the correlation between the variables in both groups, the Spearman’s correlation test was used, and after verifying the correlation between the variables of the same group. A Linear regression was performed to analyze the influence of each variable on the main characteristics of the groups. Odds ratios and confidence intervals were used to analyze the risk estimate between groups. For all analyses a p value of <0.05 was considered as statistically significant.
Results
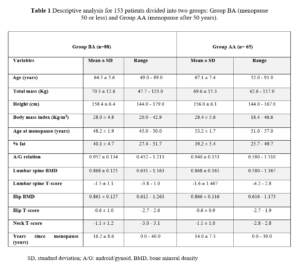
By database the study selected a total of 153 postmenopausal women aged 49 to 91 years. The sample was divided in two groups based on the menopause age: Group BA (50 or less years) or Group AA (51 to 57 years) (Table 1).
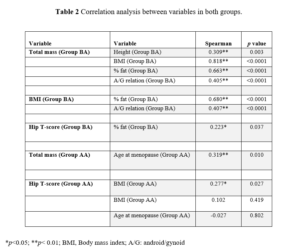
BMD was similar for the two groups, with osteopenia patterns for the spine and normal for the hip. For the two groups, neck presented with osteopenia. The age at menopause onset was on average 48.2 for Group BA and 53.2 for Group AA. Distribution test found a non-normal data distribution. However, we found no statistically significant difference for the same variables in both groups. Also, the correlation between variables in both groups was verified (Table 2).
For the BA group, the total mass (Kg) had some relation with other variables, but strong and significant correlation occurred with BMI, while for the variables height, % fat and A/G relation, total mass had a moderate and significant correlation. Regarding BMD, the T-score for hip had a significant correlation with fat percentage.
For the menopausal group over 50 years, the total mass presented a moderate and significant correlation with age at menopause onset and a weak and significant correlation with BMI. No variable correlated with BMD.
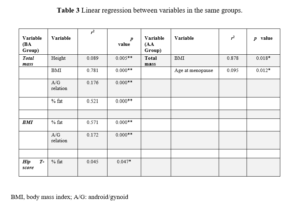
After verifying the correlation between variables in the same group, we performed a regression analysis to verify the influence of each variable over the main characteristics for groups (Table 3).
For the BA Group, the variable total mass had a great significant influence with A/G relation (17%), % fat (52%) and mainly with BMI (78%). BMI had an influence with % fat (57%) and A/G relation with (17%). Hip T-score presented influence only with % fat (4.5%).
For AA group mass also showed an important influence, but only with age at menopause (9.5%) and BMI (87%). The OR for having lower BMD in group BA was 0.82 indicating a lower risk.
Discussion
Osteoporosis is a condition that can pose the main risk for fractures. Osteoporotic fractures of the hip, spine and forearms are associated with a high degree of morbidity, leading to limited ambulation, deformities, loss of independence, and decreased quality of life. Bone loss caused by estrogen deficiency in menopause is multifactorial and complex, with biochemical changes that work in cooperation to maintain bone turnover [7].
In the present study, the participants were divided in two groups before and after 50 years. The age at menopause was on average 48.2 for the BA group and 53.2 for the AA group. One study of postmenopausal women found an association between the time since menopause, low lean and fat mass and low bone mass [8]. In our study, we found that BMI and fat percentage (% fat) displayed similar values between groups BA and AA (Table 1).
In terms of body components, for our investigation, in group BA, total mass had a moderate relation with other variables, but a strong and significant correlation occurred with BMI, while for the variables such as height, % fat and A/G relation, total mass had a moderate and significant correlation. For group AA, the total mass presented a moderate and significant correlation with age at menopause and a weak and significant correlation with BMI. However, other research has described that the greater gain in fat mass and loss of lean mass are phenomena related to the menopausal transition, and that the increase in fat mass together with the decrease in lean mass, does not seem to be different when comparing premenopausal from menopause, making weight gain imperceptible at the beginning of the menopausal transition [9].
Our regression analysis found that in group BA, total mass had a great influence with BMI, % fat, A/G relation, but only % fat had significant influence in hip T-score with a 4.5%. While for the AA group, age at menopause had an important influence on total mass (9.5%), BMI (87%), but had no influence on bone density.
Regarding relationship between age at menopause, a project that studied 756 women aged 50 to 80 years divided into 3 groups, found a relationship between lower bone mass and lower level of muscle strength in the early menopausal group when compared to the later menopausal group [10]. In a study of Tan et al. [10], regarding BMD in the perimenopause, authors found that the bone loss following 10 years is about 2-3% per year, gradually decreasing in the senile phase [11].
In this line, a study involving 897 women divided into 4 groups (premenopausal, early perimenopausal, late perimenopausal, and postmenopausal, s) based on hormone concentrations and bleeding reports, found a significant difference in femoral neck BMD in the late perimenopausal group when compared with the premenopausal one. The densitometric difference between the pre- and perimenopausal groups reached 5%; however, when examining BMD values of participants in the late perimenopausal group, about 24% of them had osteopenia, which indicates that changes in BMD already occur during this period [12]. In our investigation, we found the same characteristics, as both groups presented low BMD in femoral neck.
According to Garnero et al. [13], BMD losses during the menopausal transition can reach 10%, which means acceleration in the average of bone loss. Approximately 25% of postmenopausal women can be classified as having bone weakness [13]. Therefore, if the average age of menopause is 51 years, an early menopause would age bones proportionately. Thus, premature aging of bones would lead women to the senile phase with an already compromised skeleton [2].
Our study found that the group with a menopause onset of 50 or less years of age had a greater influence on body components and BMD of the hip, when compared to the group with onset of menopause after 50 years of age. A study analyzing the influence of time since menopause on BMD concluded that the rate of bone loss almost doubled every 5 years, with the greatest loss starting between 45 and 49 years with 3.3% and reaching 50.3% at age 85 [14]. On the other hand, Fugiel et al. [10] found that the women with early onset of menopause had a higher percentage of reduction in bone mass, muscle strength and lower and upper limb functionalities [10].
In another study regarding the influence of menopause on BMD, it was found that 30% of surveyed women over 50 years of age had osteoporosis, which is responsible for about 8% to 9% of bone fractures per year [15]. In the same line, another study reported that the greatest BMD loss occur in the first year after menopause, reaching about 5%, but may remain with losses of 1 to 1.5% in subsequent years [16]. In one study, involving 1,902 pre- or early perimenopause women at baseline, BMD of the spine and hip was evaluated. The authors found little changes in BMD during pre- or early perimenopause. However, they concluded that bone loss accelerated during late perimenopause and continued through postmenopause [17].
According to the National Health and Nutrition Examination Survey (NHANES) 2005-2008 cited by Gourlay et al. [18], the effect of osteoporosis on the femoral neck or lumbar spine is about 6.8% in women aged 50 to 59 and 34.9 % in women aged 80 years. The authors also cited a meta-analysis that analyzed 29,082 women, reporting that at the age of 65, the risk for hip fractures increased by 2.88 (95% CI 2.31-3.59) for each standard deviation [18].
In relation to the consequences of fractures, a survey presented by Svejme et al. [19] found an increased risk not only of osteoporosis but also of fragility fractures and a higher degree of mortality in women who presented menopause before the age of 47 [19]. In this line, Viswanathan et al. [20] concluded that osteoporotic hip fractures are the most important due to prolonged bed rest and limitations, accounting for about 5% of mortality, and of these approximately 21-30% die within one year of the fracture [20].
Conclusion
Evaluation of bone loss caused by each year passed after menopause is an important factor for a better understanding of postmenopausal osteoporosis. Our study found that in both groups the results were similar. The menopause group before the age of 50 years presented the highest correlations between the variables and was the group that displayed an influence of the percentage of fat on the BMD. The menopause group after the age of 50 years did not show any association between the analyzed variables and BMD.
Thus, we conclude that despite the similar results between the groups, the menopause group before 50 years of age showed the greatest associations between the variables and influence on BMD, especially in hip area.