Introduction
Increasing cesarean section (CS) rates with no obvious benefits for mothers and neonates are of worldwide concern [1,2]. Too many CSs or overuse of CS are sometimes criticized as unnecessary [2]. Women with one previous lower segment transverse CS can be good candidates for trial of vaginal birth [3] and in the majority of cases this trial is successful [4]. However, trial of labor after CS (TOLAC) has intrinsic risks for both mothers and babies [3], many obstetricians hesitate to perform TOLAC [4], and a planned repeat CS is frequently chosen. Women with previous vaginal deliveries have a very high chance of vaginal birth in the subsequent pregnancy [5], even if the previous vaginal birth was operative or a difficult one [6,7]. Mawdsley and Baskett [6] showed that women with a previous forceps delivery had more than 80% chance of a spontaneous vaginal delivery [6]. Imai showed that prolonged labor in the first delivery did recur but not frequently, and that repeat vacuum deliveries was about 5% [7]. Therefore, avoiding the first CS among nulliparous women is of paramount importance to reduce overall CS rate.
Dystocia is the leading indication for CS in the United States among nulliparous women [8]. Labor dystocia, or slow difficult labor, is known by various names such as insufficient uterine contractions, failure to progress, protracted or arrested labor, prolonged labor, dysfunctional labor, protracted or arrested descent, and cephalopelvic disproportion (CPD) [9]. The diagnosis of dystocia is also variable and subjective [10]; many CSs have been performed under the diagnosis of dystocia where, in fact, prolonged labor or difficult labor was non-existent. In 2014, the American College of Obstetricians and Gynecologists (ACOG) announced the renewed criteria for dystocia in order to safely reduce the rates of primary CS [11]. In the new criteria, longer hours with adequate uterine contraction must be allowed for a diagnosis of dystocia [11].
To reduce CS rate, it is important to know what clinical factors are associated with dystocia under strict criteria. In this study, low-risk term nulliparous women who delivered their babies vaginally and those who delivered by CS because of dystocia were compared to analyze which dystocia-related factors significantly contributed to CS.
Methods
The data of nulliparous women who delivered at the clinic between January 2004 and December 2021 were retrospectively obtained from their medical records. The inclusion criteria were delivery at ≥37 weeks of gestation, cephalic presentation, and singleton pregnancy. Women with non-vertex presentations, those with fetal demise before the onset of labor, placenta previa, abruptio placentae, morbid obesity, pathological pelvis, and uncontrolled medical diseases such as diabetes mellitus, thyroid disease, and hypertension, were excluded. Women with CS by indications other than dystocia were also excluded. The clinic in this study is located in Shizuoka City, central Japan, and the population of the city is approximately 700,000 inhabitants. Women with vaginal delivery and those with dystocia-related CS were compared with regard to eight demographic and two clinical factors as follows: maternal age, gestational age, maternal height, pre-pregnancy body weight (BW), pre-pregnancy body mass index (BMI), gestational weight gain (GWG), neonatal weight, neonatal head circumference (NHC), presence or absence of induction of labor (IOL), and pregnancy achieved by assisted reproductive technology (ART). IOL was considered when there was no onset of labor 24 hours after membrane rupture, or when the gestational age was ≥41 weeks. Oxytocin infusion was used for induction. When the cervix was unfavorable, a cervical balloon was used to ripen the cervix before oxytocin infusion. CS was performed when dilatation of the cervix and/or fetal descent was arrested for more than two hours, but there was no definite upper limit for the duration of labor [7]. This study was approved by the Local Ethical Committee (No. 21006), and informed consent was waived because of the retrospective and anonymous nature of the study.
SPSS for Windows (version 22.0; IBM Japan, Tokyo, Japan) was used for the statistical analysis. A p value <0.05 was considered as statistically significant.
Results
Data of a total of 3,059 nulliparous women were included for analysis. Data of women with non-vertex presentation (face/brow presentation) (n=3), CS because of fetal distress or abnormal fetal heart rate tracing (n=9), forelying umbilical cord (n=1), scarred uterus (previous myomectomy) (n=1) were excluded. Therefore, 3,045 nulliparous women were subject to this study, of which 2,973 women delivered vaginally and 72 women delivered by CS because of dystocia. There was one case with a fourth-degree tear. and no cases of early neonatal death. There were 7 and 2 cases of Apgar Score <7 at 5 minutes in vaginal and CS deliveries, respectively. This study included 3,031 Japanese and 14 non-Japanese women, and all of the latter delivered vaginally.
During the same period, we had 3,686 deliveries (≥37 weeks gestation, singleton) for multiparas, of which 158 delivered by CS. The indications include 124 repeat CSs, 31 breech presentations, and one dystocia. This low CS rate for multiparas has been discussed elsewhere [5].
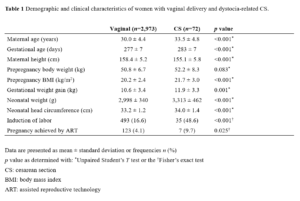
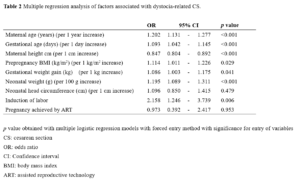
Of the 72 nulliparous women who delivered abdominally, 23 underwent CS after full dilatation of the cervix. Table 1 shows the demographic and clinical characteristics of women with vaginal and CS deliveries. Among the ten factors examined, maternal prepregnancy BW showed no significant difference between the two groups. Maternal age, gestational age, maternal height, pre-pregnancy BMI, GWG, neonatal weight, NHC, IOL, and pregnancy achieved by ART showed significant differences between the two groups in the univariate analysis (Table 1). Multiple logistic regression analysis revealed that seven factors, namely, maternal age, gestational age, maternal height, prepregnancy BMI, GWG, neonatal weight, and IOL were significant risk factors for dystocia-related CS, while NHC, and pregnancy achieved by ART were not associated with dystocia-related CS (Table 2). Duration of 1st and 2nd stage of labor in women with vaginal deliveries is presented in Table 3.
Discussion
The present study showed that among ten possible dystocia-related factors, maternal age, gestational age, maternal height, pre-pregnancy BMI, GWG, neonatal weight, and IOL were independent risk factors for dystocia-related CS in low-risk term nulliparous women (Table 2). The dystocia-related CS rate for nulliparous women in this study was low (72/3045, 2.4%). Leeman and Leeman reported an even lower dystocia-related CS rate (4/213, 1.9%) in low-risk nulliparous women [12]. This low CS rate was probably due to the clinic policy of allowing adequate time for the progression of labor (Table 3) [7].
The concept of dystocia is relatively simple: the maternal pelvis is too small, the fetus is too big, or the expulsive powers are too weak for the fetus to pass through the birth canal. However, the clinical diagnosis of dystocia is not simple. Absolute fetopelvic disproportion is uncommon, but the performance of cesarean delivery for that indication is common [13], and many CSs have been performed under a subjective diagnosis of dystocia [10]. Barber et al. [14] reported that more subjective indications (non-reassuring fetal status and arrest of dilatation) contributed to a larger proportion of the increase in primary CS than more objective indications (malpresentation, maternal-fetal, and obstetric conditions).
In addition to the above-mentioned traditional three Ps (passenger, passage, and powers), other factors are regarded to be associated with dystocia, including psyche and physician’s or hospital factors (provider factors). Anxiety and the fear of childbirth may cause dystocia. Laursen et al. [15] reported that women who feared childbirth had an increased risk of dystocia or protracted labor but not for fetal distress. When deciding to perform a CS, physicians may be influenced by the patient’s attitude, time of the day, anesthesia support, medico-legal climate, and their own training and experience [16].
Although it is generally difficult to predict the occurrence of dystocia before the onset of labor, many demographic factors have been reported as causes of dystocia-related CS, including advanced maternal age [17-20], prolonged pregnancy [19], short maternal height [17,19], maternal obesity [17-19,21], weight gain during pregnancy [19], neonatal weight [19], and neonatal head circumference [22]. Hautakangas et al. [17] reported that a 1 kg/m2 increase in maternal pre-pregnancy BMI increased the CS rate by 10%. Chen et al. [19] showed that every 3 kg/m2 increase in BMI increased the CPD-related CS rate by 1,320 [19], which means a 1 kg/m2 increase in BMI increased the CS rate by 9.7%. The current study showed that every 1 kg/m2 increase in pre-pregnancy BMI increased the CS rate by 11.4% (Table 1), which was a little higher than the rated found in the aforementioned two reports [17,19].
In the present study, GWG as well as pre-pregnancy BMI were associated with dystocia-related CS (Table 2). This suggests that controlled weight gain during pregnancy is also important for avoiding dystocia-related CS. Wu et al. [18] stated that maternal BMI at labor, not prepregnancy BMI, was more representative of the maternal mechanical status in labor. Chen et al. [19] showed that in nulliparous women, CPD was higher for shorter, older, and more obese women, with large weight gains, larger fetal birth weights, and longer gestational ages, which is consistent with our study. However, they considered only demographic factors, and the effects of IOL, ART achieved pregnancy, or other clinical factors related CPD were not analyzed.
ART pregnancy is considered to be associated with dystocia. Sullivan et al. [23] reported an overall CS rate of 44% in nulliparous women who became pregnant through ART. The “precious baby” concept may influence clinicians’ decision-making for CS in ART achieved pregnancies. However, the current study did not show such an association between ART achieved pregnancies and dystocia-related CSs in the multiple regression analysis (Table 2). In our clinic, pregnancies achieved by ART do not change the policy on how to manage labor and delivery. The author agrees with Sullivan et al. [23] in that vaginal delivery should be supported in women achieving pregnancy through ART and the indications for CS should be evidence-based.
The relationship between IOL and CS has been controversial. Mishanina et al. [24] concluded in their meta-analysis that women whose labor was induced were less likely to have CS than those managed expectantly. However, Levine et al. [25] stated that IOL increased CS by approximately three times, irrespective of parity. Davey et al. [26] also reported that in nulliparous women, IOL increased CS rate by 2.5 times. Observational studies using Robson ten-group classification system showed that in many countries Robson group 2a (nulliparous, singleton, cephalic, ≥ 37 weeks, induced labor) had consistently higher CS rates than Robson group 1 (nulliparous, singleton, cephalic, ≥ 37 weeks, spontaneous labor) [27,28]. Our study showed that IOL increased dystocia-related CS by 2.1 times (Table 2). One of the reasons for this discrepancy concerning IOL and CS rate is different definition of expectant management [29]. We postulate that the need for IOL might already be a sign of dystocia. As both prolonged gestational age and IOL increase CS rates (Table 2), whether nulliparous women at 41 weeks of gestation with unfavorable cervix should receive IOL remains a challenging clinical issue.
The effect of epidural analgesia on dystocia is also a controversial issue. In the current study, the relationship between the use and non-use of epidural and dystocia was not analyzed because epidurals were used more frequently in nulliparous women with already prolonged labor (a large selection bias already existed). Goer has stated, “at the very least we cannot assure women with confidence that epidurals don’t increase the likelihood of cesarean” [30].
Of the seven factors shown to be associated with dystocia in our study (Table 2), maternal age and maternal height are unmodifiable. However, pre-pregnancy BMI and GWG can be controlled, and neonatal weight has a positive relationship with maternal weight gain [31], suggesting that good weight control before pregnancy and appropriate weight gain during pregnancy might decrease dystocia-related CSs.
Recently, computed tomography and magnetic resonance imaging have been introduced for pelvimetry to assess the adequacy of the maternal pelvis [32,33]. These methods can measure pelvic diameter more precisely and are useful, especially in cases of vaginal breech and twin delivery [33]. However, dystocia occurs not simply because of a narrow bony pelvis. The fetal head is an excellent pelvimeter and CPD can only be diagnosed with assurance during labor. Intrapartum ultrasound is another new method for evaluating the progression of labor [34]. As ultrasound is more precise than clinical digital examination for assessing fetal position and station, the use of ultrasound for the management of labor may reduce the subjective diagnosis of dystocia and CS use.
Our study has some limitations. First, it exclusively included Japanese women. Since the progression of labor and delivery have racial differences [35], the results may not apply to non-Japanese women. Second, this study did not analyze other possible dystocia indicators, such as maternal mental condition [15] and the use or non-use of epidurals [31].
However, this study has several strengths. The diagnosis of dystocia and decision for CS are quite different among physicians and hospitals [16]. In our study, the diagnosis of dystocia and decision to perform a CS were made by a single doctor, which precluded such inter-physician and inter-hospital variations. A longer time was allowed to avoid unnecessary intervention for slowly progressing labor (Table 3), therefore, the dystocia-related CS rate was low, and this study may help to elucidate non-subjective dystocia-related factors precisely.
In conclusion, this study showed that gestational age, maternal age, maternal height, pre-pregnancy BMI, GWG, neonatal weight, and IOL were significantly associated with dystocia-related CS among low-risk term nulliparous women. Well-controlled weight gain during pregnancy and the careful use of IOL may reduce dystocia-related CS in term nulliparous women.
Research funding: None declared.
Author contributions: The author has accepted responsibility for the entire content of this manuscript.
Competing interest: The author states no conflicts of interest.
Ethics approval and consent to participate: This study was approved by the local ethics committee (No. 21006). Our ethics committee does not require informed consent for retrospective observational studies as long as anonymity is protected.
Acknowledgments: The author is grateful to the midwives, nurses, and coworkers in the clinic, to Yousuke Sasaki (Department of Medical Statistics, Satista Co. Ltd, Uji, Japan, www.satista.jp) for his assistance with the statistical analysis, and to Editage (www.editage.com) for the English language editing.
Data availability: The data that support the findings of this study are available from the corresponding author upon reasonable request.