Introduction
Congenital hypogonadotropic hypogonadism (CHH) is a rare disease in which insufficient or deficient secretion of gonadotropin-releasing hormone (GnRH) causes delayed or non-development of puberty and infertility. It is more common in males by a ratio of five to one [1]. The higher incidence of CHH in men has led to women often being ignored in previous studies. Accompanied by the presence or absence of normal olfaction, it can be divided into normosmic hypogonadotropic hypogonadism (nHH) and Kallmann syndrome (KS) [2]. CHH patients show low or normal serum levels of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) [3]. CHH can affect a patient’s reproductive system, bones and psychology [4]. Thus, early and timely diagnosis and treatment are necessary. We report on a female CHH patient who delivered a healthy baby after hormone replacement therapy (HRT) and pulsatile GnRH treatment.
Case report
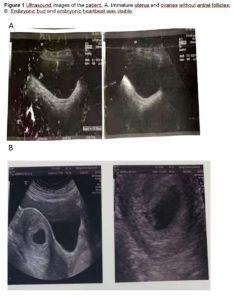
In 2009, a 25-year-old woman presented without menarche. Serum sex hormones were FSH 0.28 mIU/mL, LH 0.00 mIU/mL, estradiol (E2) 0.00 pg/mL, progesterone (P) 0.00 ng/mL, prolactin (PRL) 3.01 ng/mL and testosterone (T) 20.67 ng/mL. We performed a pelvic ultrasound which showed an immature uterus, right ovary 1.6 cm, left ovary 1.1 cm and two small follicles were faintly seen (Figure 1A). A pituitary MRI was performed which suggested that the pituitary gland was slightly smaller. The patient had a normal sense of smell and no systemic disease. Her physical examination revealed that breast tissue was bilaterally palpable outside the areola without areola development (Tanner stage 2 for breast [5]), no pubic hair and the labia minora had not developed sufficiently. The patient was diagnosed with CHH and managed with HRT with a sequential combination of 2 mg estradiol valerate and 1 mg cyproterone acetate. Menstrual bleeding occurred after one cycle of medication. One year after taking medication, serum endocrine hormones were assessed: FSH 0.29 mIU/mL, LH 0.00 mIU/mL, E2 94.64 pg/mL, P 0.63 ng/mL, PRL 5.44 ng/mL, T 25.58 ng/mL, and thyroid stimulating hormone (TSH) 5.12 mIU/L. After 4 years of treatment, the HRT was switched to an oral sequential combination of 2 mg 17 beta-estradiol/10 mg dydrogesterone. Menstruation was regular during HRT use.
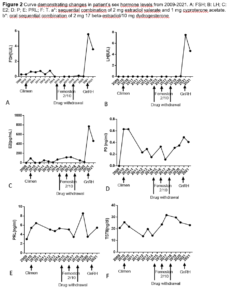
The patient married at 35 years of age and wished to conceive a baby. In our department, transvaginal ultrasound monitoring was started to detect ovulation. However, her follicles only developed slowly without dominant follicles. In 2021, a pulsatile GnRH pump was used with gonadorelin at a dose of 12 µg/90 min. Since no obvious follicles were detected during transvaginal ultrasound, the dose was gradually adjusted to 15 µg/90 min. Within the two next consecutive cycles, dominant follicles were observed, but no pregnancy occurred. On May 14 2021, we found that the largest follicle on the right side was 1.85 x 1.55 cm. Laboratory data showed: FSH 3.6 IU/L, LH 4.63 IU/L, E2 402.99 pg/mL, P 0.41 ng/ml, PRL 5.48 ng/mL, T 23.14 ng/mL (Figure 2 shows sex hormone levels from 2009 to 2021). On May 31 2021, the patient’s urine β-hCG was positive. She was provided with luteal support with dydrogesterone and we took her off the pulsatile GnRH pump. Blood results from June 18 2021 were β-hCG 98,073.28 IU/L, P 30.66 ng/mL, PRL 7.67 ng/mL and E2 922.61 pg/mL. Ultrasonography showed that the uterus was anterior, the size of uterus was 6.6 x 5.3 x 4.4 cm, the muscular layer echo was homogeneous, the pregnancy sac was visible in the uterine cavity with a size of 2.3 x 2.7 x*1.8 cm. A 0.7 cm embryonic bud was seen in the uterus and embryonic heartbeat was visible. Examination hints were a live intrauterine pregnancy with an ultrasound gestational age of 6 weeks and 4 days (Figure 1B). Dydrogesterone was discontinued at 12 weeks of pregnancy and a 3,150-gram male child was delivered after normal labour at 37 weeks of pregnancy.
Discussion
We herein report on a 25-year-old woman diagnosed with CHH presenting with primary amenorrhoea. HRT was used to develop secondary sexual characteristics and maintain normal hormone levels. At the age of 35, she married and desired to have a baby. Pulsatile GnRH 15 µg/90 min was used and five months after being treated, the patient became pregnant and gave birth to a healthy baby boy.
Puberty is a necessary stage in the transition from childhood to adulthood. It is the process by which the biological individual moves toward sexual maturity and the acquisition of reproductive capacity [6]. Physiologically, the initiation of puberty is marked by the pulsatile release of GnRH in the hypothalamus, gradually forming regular GnRH pulses with a frequency of about one pulse every 60-90 minutes. The pulsatile secretion of GnRH stimulates the pituitary gland to secrete LH and FSH, constituting a positive and negative feedback system for the hypothalamic-pituitary-gonadal (HPG) axis [7]. The term delayed puberty refers to the delayed onset or progression of pubertal development ranging more than two standard deviations from the population average. There is a transient GnRH deficiency that causes constitutional delay of growth and puberty [8]. Conversely, CHH is a rare condition in which women suffer from a chronic GnRH deficiency. This results in reduced levels of E2, FSH and LH and consequently a partial or complete absence of puberty, amenorrhoea and infertility. CHH has been classified as KS or nHH according to whether it is combined with olfactory impairment. The prevalence of CHH in women is low and can easily be overlooked.
Some studies have identified that women with CHH are generally diagnosed late and have poorer adherence to treatment and higher levels of depressive symptoms [1,4,9]. It is critical to diagnose and treat CHH correctly at an early stage since it negatively impacts the patient's mental and physical health. CHH is a diagnosis of exclusion, and it is crucial to distinguish it from other causes of primary or secondary amenorrhoea. In addition to assessing sex hormones, other diagnostic tools include a pelvic examination, abdominal ultrasound, MRI scan of the brain, a detailed history of dietary habits, family history, eating disorders, exercise, weight, development, menstruation, mental distress, bone density tests, thyroid hormone tests and GnRH stimulation tests [10]. The main goal of CHH treatment is to provide exogenous GnRH or HRT [11]. This is dependent on the timing and purpose of treatment for puberty induction, general health or fertility.
The pulsatile GnRH pump is an effective treatment method that conforms to the physiological regulation mechanism of the HPG axis. A miniature GnRH input device controlled by artificial intelligence was used to simulate the physiological pulse secretion pattern of GnRH in the hypothalamus by pulsed subcutaneous injection of GnRH analogues. This is a method to stimulate the pituitary gland to secrete gonadotropins, which in turn leads to the development of the gonads, the production of sex hormones and the acquisition of fertility. This technique is currently used in the treatment of CHH and central secondary amenorrhoea, and has been used with some success in women with CHH who have fertility requirements [12,13]. Leyendecker et al. proposed the use of pulsed GnRH for CHH patients. Pulsatile GnRH induces ovulation in patients with CHH [14]. GnRH pump therapy is associated with a risk of ovarian hyperstimulation; therefore, supervision is required during the treatment process [15].
Conclusion
Women with CHH often present with amenorrhoea, as well as with or without gonadal dysplasia or normal secondary sexual characteristics. The main aim of treating CHH is to induce and maintain normal pubertal development and to prevent prolonged hypoestrogenism from affecting fertility. Early diagnosis and long-term management of CHH patients in women are important. The patient in this presented case was treated with HRT, which promotes secondary sexual characteristics and maintains normal hormone levels. Ovulation induction and pulsatile GnRH therapy are required when the patient requires fertility treatment. This successful case will provide ideas for the future treatment of women with CHH.
Funding: This work was supported by the Beijing Municipal Health Commission and the Beijing Municipal Administration of Hospitals’ Ascent Plan (No. DFL20181401) provided to Xiangyan Ruan.
Informed consent statement: Not applicable.
Acknowledgments: Not applicable.
Conflicts of Iinterest: The authors declare no conflicts of interest.