Introduction
According to the World Health Organization (WHO), from 1990, worldwide cesarean section (CS) rates have risen and continue to increase, currently accounting for more than 1 in 5 (21%) of all childbirths. Although CS is recognized as an important way to protect maternal and fetal health, in some critical situations, unnecessary surgical procedures can be harmful for both [1].
The WHO recommends that CS rate should be lower than 10% [2] because it has been observed that even a with a CS rate below 10% there is a decrease in maternal and neonatal mortality rates [3].
In Europe, between 2010 and 2018, the estimated CS rate was 25.7% (confidence interval [CI] 23.4 to 28.0) [4]. In Italy, data from 2020 revealed an average CS rate of 31.3%, with a higher incidence in specific regions and in affiliated private clinics, and a lower rate in public institutions [5].
Since the 1970s and 1980s, some clinicians started to register data relating to trial of labor after cesarean delivery (TOLAC) and concluded that if properly conducted, a vaginal delivery after CS is relatively safe [6,7]. Current guidelines recommend to counsel and offer a TOLAC to the majority of women with one previous CS with a low-transverse incision, with or without a history of previous vaginal delivery (PVD) [8-11].
Regarding Italian data, only 11.2% of women in 2020 with a history of a previous CS have had a vaginal birth after cesarean section (VBAC) [5]. In terms of benefits and risk, VBACs may decrease the risk of surgical related complications. Regarding the risk of transfusion, thromboembolic disease, hysterectomy and endometritis, controversial data emerges between patients having a VBAC and repeated caesarean section [8,9,12,13]. However, some authors agree that the greatest risk of adverse outcome occurs in a TOLAC resulting from an emergent cesarean delivery [8-10]. Uterine rupture or dehiscence of the uterine scar are the most feared risks associated with a TOLAC and the reported incidence may vary according to the literature from 0.5 to 2.9% [12,14-16]. Although maternal or neonatal/infant death is considered a rare consequence of a uterine rupture, it has been described in the literature. Moreover, other adverse outcomes may occur such as severe hemorrhage, neonatal pathological acidosis, hysterectomy, and/or damage to the genitourinary tract [17-19].
Due to the varying complication rates related to TOLAC described in literature, we decided to perform an analysis of a population with a singleton pregnancy in cephalic presentation undergoing TOLAC in terms of maternal and neonatal outcomes and determine factors associated with uterine rupture and uterine scar dehiscence.
Methods
This was a monocentric retrospective observational study regarding TOLACs performed during 2016 and 2019, at the Santa Maria della Misericordia University Hospital in Udine, Italy. Institutional Review Board approval of this study was obtained from the DAME (Italy), Prot IRB: 067/2021.
All women who had been declared eligible for TOLAC according to our department protocol were included in the study. In contrast, women who opted for elective repeat cesarean delivery (ERCD), or who, upon admission, underwent an urgent CS were excluded. Inclusion criteria were: cephalic singleton pregnancy at 24-42 weeks of gestation; one or two previous cesarean sections performed at the level of the lower uterine segment documented in surgical records or with an unknown type of prior uterine incision, without clinical suspicion of a previous classic longitudinal uterine incision; previous myomectomy without opening of the uterine cavity. Exclusion criteria included: three or more previous CSs; previous transfundal uterine incision; previous T or J incision; history of previous uterine rupture or uterine scar dehiscence; and/or contraindications to vaginal delivery. The following were not considered exclusion criteria: number of uterine suture layers (single vs double) in the previous CS; labor dystocia as indication for the previous CS; indication for induction of labor; maternal pre-pregnancy body mass index (BMI) ≥ 30; pre-gestational or gestational diabetes; fetal growth below the cut-off for which elective CS is suggested (estimated fetal weight ≥ 5,000 g in non-diabetic women and ≥ 4,500 g in women with diabetes). The gestational age did not influence the eligibility for a TOLAC. Patients with a previous CS were informed about the benefits and complications related to TOLAC or an ERCD, and together with the obstetrician evaluated their birth options. Patients were provided with written information regarding the risks/benefits of both modes of birth to support delivery decision-making. Checklists were used for documentation of counseling and management.
Demographic and obstetrical data were obtained by analyzing both paper medical records and the Insiel G2 computer system. The data were made anonymous and prepared for analysis. Patients who underwent a TOLAC were subject to continuous electronic fetal monitoring recording. Induction of labor, if indicated, was performed via one or more of the following modalities: Cervical Ripening Balloon (CRB), amniorhexis or oxytocin infusion. The choice of induction modality was based on the Bishop's cervical score upon admission.
Our primary outcome was the mode of delivery, i.e., whether TOLAC results in a VBAC or an emergent. Secondary outcomes related to maternal and neonatal outcomes. Maternal health outcomes included uterine rupture, uterine scar dehiscence, hemorrhage and/or need for blood transfusion, or peripartum hysterectomy. Uterine rupture was defined as full thickness separation of all layers of the uterine wall. Uterine scar dehiscence was defined as a disruption of the uterine muscle with intact overlying peritoneum. Before discharging the patient home, we performed a pelvic ultrasound to investigate occult uterine rupture/uterine scar dehiscence.
Neonatal outcomes included Apgar scores at 5 minutes <7, admission to the neonatal intensive care unit (NICU), and neonatal death (defined as any death occurring from the birth until discharge from the hospital).
Statistical analysis was performed using R Software (version 3.6.3- www.R-project.org). The Kolmogorov-Smirnov test was used to test for normality of the continuous variables. Continuous variables were compared with the use of the Student’s T -test, the Wilcoxon test, one-way ANOVA, or the Kruskall-Wallis test. The categorical variables were compared using chi-square or Fisher’s exact test. Multivariate and univariate logistic regression analysis were performed to adjust for potential confounding factors for the composite end point of the rate of uterine rupture/uterine scar dehiscence. A p value <0.05 was considered as statistically significant.
Results
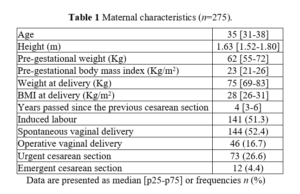
The study population included 275 patients who were candidates for TOLAC and gave consent to participate. Characteristics of patients who undertook TOLAC are reported in Table 1. 17 women had a history of uterine surgery without opening of the uterine cavity, primarily myomectomies, of which 6 had then performed at least one delivery by CS following this surgery. 250 women had undergone only one CS, whereas 14 women had undergone two cesarean section deliveries. 84.0% of women had never experienced a vaginal delivery either before or after CS. At admission, median Bishop score for all patients was of 4 points (p25-p75: 2-6), with a median cervical dilation of 1.5 cm (p25-p75: 0-3) and a median cervical effacement of 50% (range: 15-80%). 141 patients (51.3%) underwent induction of labor, with 15 cases of failed induction and the need to proceed with a CS. 69.0% of patients required placement of a catheter for epidural anesthesia. The gestational age at admission ranged to 32 - 41 weeks of gestation. Of the total population, 69.1% had a vaginal delivery, i.e., a successful TOLAC with VBAC; the remaining 30.9% underwent a repeat intrapartum CS. Considering the patients who gave birth vaginally, in 52.4% of cases it was a spontaneous vaginal delivery, while in the remaining 16.7%, it was necessary to proceed with a vacuum-assisted operative vaginal delivery (OVD).
There were 16 CS (4 emergent and 12 urgent) whose indication was a suspected uterine rupture or dehiscence, mainly based on the patient’s declaration of severe localized pain at the site of the previous hysterotomy. Of these, in 11 cases, the presence of uterine rupture or dehiscence of hysterotomy scarring was confirmed during the surgery. Table 2 shows indications for OVD, urgent cesarean (UC: maternal or fetal compromise, no life threat) and emergent cesarean (EC: immediate maternal/fetal life threat). No uterine rupture or dehiscence was found in the VBAC group.
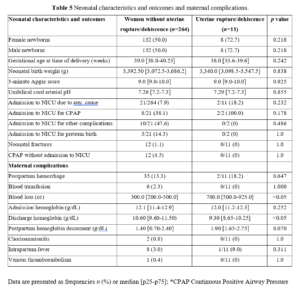
The median infant Apgar score at the fifth minute was 9.2 (n=275); an 8.4% required admission to the NICU, of which 43.5% required prolonged treatment with CPAP. A transfontanellar brain ultrasound was performed in 9 of the 275 infants during NICU admission. Of these, 78.0% were normal, while 2 infants had mild ultrasound abnormalities such as mild periventricular hyperechogenicity and cortical brightening on day 1 that resolved on day 3.
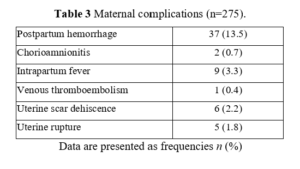
Maternal complications are presented in Table 3. In 6 cases of the 37 patients who had postpartum hemorrhage, a blood transfusion was required, with a median of 2 units of packed red blood cells per patient. No hysterectomies or maternal deaths occurred in this series. We compared maternal characteristics between women with uterine rupture/dehiscence and women without uterine rupture. 11 patients with uterine scar dehiscence or uterine rupture did not present statistically significant differences in maternal characteristics compared to patients without uterine rupture. 9 patients had a history of a single uterine incision following CS, 1 patient had a history of two cesarean sections, and 1 patient had a history of laparoscopic myomectomy of an intramural myoma. Exploring the indications of previous CSs in the two populations, none presented statistical significance. The time interval between uterine surgery and TOLAC in the group in which a uterine rupture or dehiscence occurred was not statistically different from that of patients in which this event did not occur, with a median interval of 4 years and 3.5 years, respectively, with a p value of 0.83. All 11 patients had never experienced a prior vaginal delivery.
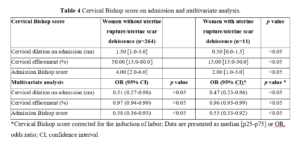
Uterine rupture or dehiscence were more likely to occur in women with an unfavorable cervix on admission than women with a favorable cervix. This difference regarding the status of the cervix was statistically significant, as reported in Table 4. Conversely, induction of labor did not show a statistically significant correlation with uterine rupture or dehiscence of the uterine scar. Regarding neonatal characteristics and outcomes, no statistically significant differences were found between the two groups (Table 5). Regarding maternal complications (Table 5), results regarding blood loss during delivery were significant. Despite this, none of the patients with uterine rupture or dehiscence required transfusion of concentrated blood products.
Discussion
The success rate of TOLAC in our study population is superimposable to that found in the literature with a VBAC rate of 69%, already highlighted by the review of Sabol et al. [20]. This result appears even more promising considering that the study population consisted of 84.0% of patients who had never experienced a vaginal delivery, a factor that decreases the chance of a VBAC outcome compared to patients with at least one vaginal delivery in their obstetric history [21,22]. In fact, the literature reports a VBAC rate of 83.0% for women without a history of PVD and 94% for women with a history of PVD [23].
In our study population there were 5 uterine ruptures and 6 hysterotomy scar dehiscences. Respectively, these events affected 1.8% and 2.2% of the study population for a total of 4.0%. This higher rate of uterine ruptures, as compared to the literature [12,14], is affected by the extreme discordance in the definition of uterine rupture and dehiscence within the literature itself. In fact, some studies rely on anatomical criteria alone to define uterine rupture and distinguish it from a dehiscence, while others rely on clinical evidence suggestive of maternal (vaginal bleeding, acute pain at the level of the previous hysterotomy scar, hemodynamic instability) 8 and fetal complications (fetal monitoring alterations) [24,25]. There are still some authors who do not distinguish the two entities and instead enclose them into a single outcome called uterine rupture. This makes it extremely difficult to compare results between different studies. Other factors that may influence the higher percentage of uterine ruptures in the study population include: a high percentage (84%) of patients who did not have any PVD (a history of PVD, whether before or after CS, is associated [12] with a reduced risk of uterine rupture); and a numerically small sample compared with reference studies. Guise et al. [12] constructed a review analyzing 203 articles, thus ensuring a rather relevant sample size.
Despite the higher incidence of uterine rupture of our series as compared to the literature, there was no evidence of any serious adverse outcome our patients. Guise et al. [12] in their analysis found that the main maternal outcome of a uterine rupture is a hysterectomy (14 to 33%), finding no associated maternal deaths. In our study, no maternal death occurred in women who had a uterine rupture, a finding aligned with the literature [12,26], and a CS with hysterectomy was never necessary. Patients with uterine rupture did not require concentrated blood transfusions, testifying a modest extent of blood loss and good control of postpartum hemorrhage in the group with uterine rupture. Regarding blood loss during delivery, this was statistically significantly higher in the rupture group (700 cc in the group with rupture/dehiscence versus 300 cc in the group without this complication, with a p value <0.05). This finding is justified by the fact that all patients with suspected uterine rupture or dehiscence underwent an intrapartum CS, a procedure that involves greater blood loss than a vaginal delivery. This fact also justifies a lower hemoglobin concentration at discharge, which was also statistically significant (9.3 g/dL vs 10.6 g/dL, with a p value <0.05) in the group of patients in whom uterine rupture/dehiscence occurred.
With regard to neonatal outcomes, the rate of perinatal mortality associated with uterine rupture reported in the literature varies from 5 to 26% [14,27,28], while the incidence of neonatal hypoxic-ischemic encephalopathy associated with uterine rupture is around 6% according to Landon et al. [26], with the presence of severe forms of hypoxic-ischemic encephalopathy [28]. In our series, among patients who reported uterine rupture or dehiscence, there were no neonatal deaths, while 2 infants (18.2%) required admission to the NICU due to a mild hypoxic-ischemic encephalopathy that left no evident trace in one of the two infants, while the other infant presented a mild axillary hypotonia at discharge. Our data, therefore, shows that uterine rupture is associated with an absence of highly unfavorable neonatal outcomes such as neonatal mortality and severe hypoxic-ischemic encephalopathy.
Contrary to previous studies [14,26,29,30], in our study population induction of labor was not statistically correlated with an increased risk of uterine rupture. In our population, even a low Bishop score appears to be associated with an increased risk of uterine rupture or dehiscence. In fact, patients who reported uterine rupture/dehiscence had a median Bishop score on admission of 2 points.
In conclusion, this present study confirms, first of all, the success rate of TOLAC. Even more the reassuring information that emerges from the collected data collected indicates the extreme safety of the trial of labor, not only because of the VBAC outcome, but also in the case of the onset of the most feared complication in this patient population, namely a rupture of the uterus. Although a high rate of uterine dehiscence and rupture was observed, the absence of serious outcomes, both maternal and perinatal, recorded in the study population is certainly an encouraging finding that favors an increased use of TOLAC in candidate patients.
The main limitation of the study was the small sample size. In addition, we must consider that, although Bishop's score is the most widely used tool for the evaluation of the chances of vaginal delivery, it remains a subjective method with considerable operational variability, that not always is reliably replicated.