Introduction
Menopausal hormone therapy (MHT) has been a source of controversy since its introduction. Firstly, estrogens alone were used, later observing an increased risk of endometrial cancer. Later, it was reported that by associating progesterone endometrial risk was reduced. There was a hormone boom that lasted for a couple of decades, until the Women's Health Initiative (WHI) study caused its use to virtually die out [1]. However, climacteric women continued to have symptoms that severely deteriorated their quality of life and presented metabolic disorders that increased the risk of chronic diseases, thereby paying a high cost in their physical, mental, and social well-being [2,3]. The objective of this review is to substantiate the reasons why doctors decide to prescribe MHT to their patients.
Quality of life
The World Health Organization (WHO) defines Quality of Life as the perception that an individual has of his/her situation in life, in relation to objectives, expectations and interests [4]. This definition was later also applied specifically to health, defining the concept of Health-Related Quality of Life as the evaluation that a patient makes of the impact that a health condition and its treatment have on his/her daily life [5].
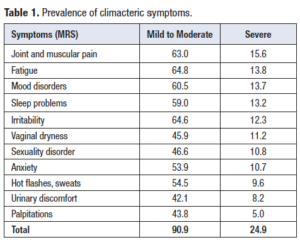
The climacteric has been associated with a series of symptoms that, if they reach a certain intensity, can severely impair the quality of life. A study carried out in Latin America in 8,373 women between 40 and 59 years of age, to whom the Menopause Rating Scale (MRS) was applied, showed a high prevalence of climacteric symptoms [6]. In Table 1 one can observe that 90.9% of the studied population had at least one climacteric symptom and in 24.9% of these women some of these symptoms were severe (symptom score greater than 2 on the MRS scale).
Osteoarticular discomfort, physical and mental fatigue, and depressed mood were among the most frequently reported symptoms; the intensity of these symptoms was severe in 15.6%, 13.8% and 13.7% of women, respectively. Osteoarticular disorders represented the most prevalent severe symptom in this group of women and this observation is compatible with the fact that this symptomatology is responsible for 44.3% of female work absenteeism in a Chilean hospital [7]. In the Netherlands, chronic musculoskeletal pain costs approximately 20 billion euros per year (direct and indirect costs) [8]. A study carried out in Finland determined that general practitioners request laboratory tests for 12% of patients with musculoskeletal discomfort, images for 24%, with 61% of them being prescribed analgesics, 16% go to the kinesiologist and 7% are referred to the specialist [9]. All this implies an overload to healthcare systems. In the previously mentioned study [6], in addition to musculoskeletal discomfort, vasomotor symptoms (i.e hot flashes and diaphoresis), most related to climacteric, had only a 9.6% prevalence.
Sexuality is an important aspect of a woman's quality of life and is reflects her physical, psychological and social well-being [10]. During the climacteric, genitourinary syndrome, mood disorders, and irritability can affect female sexuality. A study that applied the Female Sexual Function Index (FSFI) to 7,243 otherwise healthy women aged 40 to 59 of 19 health centers in Latin America, showed that 56.8% of them had sexual dysfunction and 25.6% had not been sexually active in the past year. The main risk factor associated with sexual failure was poor vaginal lubrication (OR: 3.86; 95% CI 3.37 to 4.43) [11].
If we consider that the concept of quality of life is centered on the perception of the patient, climacteric symptoms, when defined as severe, will affect the woman's quality of life [2]. This symptomatology increases as ovarian function declines and with it, quality of life is progressively compromised. According to the MRS, there is a severe compromise in quality of life (MRS total score >16 points) in 12.9% of premenopausal women aged 40 to 44, a figure that increases to 26.4% in perimenopausal women, 31.6% in the early postmenopausal and 29.9% in late postmenopausal women (> 5 years) [6].
This deterioration in quality of life, as a consequence of climacteric symptoms, can be reversed with MHT. A meta-analysis shows that MHT reduces the frequency of vasomotor symptoms by 77% and their intensity by 87% [12]. Despite this, MHT not only improves hot flashes, but also other climacteric complaints such as mood swings, osteoarticular pain, poor quality of sleep, among others. A US study of 459 women assessed for 1 year with the Women's Health Questionnaire, the 15D questionnaire, and a climacteric visual analogue scale, found that continuous combined estrogen-progestin therapy produced significant improvement quality of life, by the 12th week of treatment, and that this improvement increased up to week 52 of the study in all domains of quality of life [13].
Chronic conditions
Weight gain and obesity
Obesity is an important factor that could increase the risk of developing chronic conditions during the climacteric. In this period, neurochemical disorders of the nervous system associated with estrogen deficiency, are not only involved in the etiopathogenesis of climacteric symptoms, yet also in changes in body composition, especially in the increase in adipose tissue, a central factor in the etiopathogenesis of chronic diseases. Thus, for example, there is evidence that supports a common etiology for mood disorders and obesity, with cell signaling mechanisms being the main ones involved, since these signals modulate energy balance and the stability of the body. Leptin, for example, has many actions within the brain, including the reduction of food intake and increasing energy expenditure. In addition, in climacteric women, hypoestrogenism alters the sensitivity to leptin at the level of the central nervous system (CNS) level and consequently modifies the distribution of body fat [14]. Morris et al. [15] have pointed out that, in obesity, rather than a leptin deficit, there is a resistance to its action and, consequently, a decrease in its signals, which would lead to a greater food intake and a decrease in caloric expenditure. The authors conclude that leptin is associated with depression and that this relationship seems to be mediated by an increase in adiposity [15]. However, leptin is not the only actor that plays an important role in the mood disorders and in obesity, there are also other signals involved: oxygenic and anorexigenic neuropeptides, metabolic factors, stress hormones, cytokines, and neurotrophic factors [16]. After menopause many of these metabolic signaling pathways are altered. Hypoestrogenism, in addition to altering leptin sensitivity, associated with increased adiposity, is associated with an increase in oxygenic neuropeptides (neuropeptide Y, ghrelin, and melanin-concentrating hormone) [17] and a decrease in anorexigenic neuropeptides (insulin, leptin and serotonin). It has also been described that mood disorders are associated with a sedentary lifestyle, another factor that increases the risk of obesity (OR 1.50; 95% CI 1.30 to 1.53) [18]. Therefore, estrogen deficiency influences many mechanisms that could explain weight gain observed in middle-aged women and, with it, the increased risk of chronic conditions in climacteric women.
Cardiovascular disease
In recent years we have increased our understanding of the mechanisms through which abdominal obesity increases cardiovascular risk. These concepts have focused on a new paradigm known as the metabolic syndrome, a condition characterized by abdominal obesity associated with the presence of cardiovascular risk factors, such as glucose and lipid metabolism disorders, and high blood pressure [19]. In its etiopathogenesis, the central role appears to be played by the increase in abdominal fat, which leads to an increase in the production of a series of pro-inflammatory proteins and a decrease in anti-inflammatory proteins, which, acting through endocrine, autocrine and paracrine mechanisms, lead to a chronic inflammatory state, thereby increasing cardiovascular risk. These mediators include tumor necrosis factor α, leptin, adiponectin, resistin, PAI-1, interleukin-6, angiotensinogen, serum amyloid A, and C-reactive protein [20]. There is a relationship between obesity, diabetes mellitus and cardiovascular disease. In the obese patient, the adipocyte modulates the secretion of a series of adipocytokines such as resistin, leptin, adiponectin, among others, which will influence the function of pancreatic beta cells, and even their survival. If we add to this the fact that resistin blocks insulin receptors, one can understand the central role of adipocytes in the etiopathogenesis of diabetes, one of the main cardiovascular risk factors [21].
It has been reported that not only abdominal fat is associated with increased cardiovascular risk, but also that adipose tissue surrounding the heart is associated with increased coronary risk [22]. Cardiac adipose tissue could locally modulate the morphology and function of the heart and blood vessels, through the production of cytokines, which could possibly have a role in cardiac adiposity-related atherosclerosis. The amount of epicardial adipose tissue has been shown to be significantly related to carotid intima media thickness, an index of subclinical atherosclerosis. A clear relationship has been pointed out between menopause, endogenous estrogen levels, heart fat, and cardiovascular risk, which is one more reason to avoid weight gain at this stage of life [23].
But not only obesity increases cardiovascular risk in postmenopausal women, but also estrogen deficiency per se is a determining factor in the development of atherosclerosis. Estrogen decreases cell apoptosis and increases resistance to injury, decreases the passage of plasma LDL toward the endothelium, is an antioxidant, decreases monocyte adhesion, monocyte chemotactic response, and smooth muscle cell proliferation [24]. Therefore, it is not surprising that an almost three-fold increase in the extent of atherosclerotic plaques in the aorta is observed in castrated rabbits fed with a hypercholesterolemic diet, a situation that is not observed in non-oophorectomized rabbits [25]. Another mechanism that could be at play in the increased cardiovascular risk is the loss of vasodilation mediated by type II angiotensin receptors, which would be modulated by estrogens and mediated via nitric oxide, generated by endothelial nitric oxide synthetase and by endothelium-derived hyperpolarizing factor. This vasodilatory effect requires estradiol and the presence of XX chromosomes [26].
Before the publication of the WHI study, there was a general idea in many observational studies that MHT decreased cardiovascular risk. However, the WHI reported that combination hormone therapy increased the risk of coronary heart disease by 29% and cardiovascular events by 41% [27]. These surprising results were attributed to the use of oral therapy in older women who probably already had atherosclerotic lesions, in whom the use of oral ovarian hormones increased thrombotic risk due to instability of atherosclerotic plaques [28]. This hypothesis seems to be confirmed in an analysis of the Nurses' Health Study, which found that women who started combination MHT in the first 10 years after menopause had a relative risk (RR) of coronary heart disease (CHD) of 0.72 (95% CI 0.56 to 0.92), while those who started it later had a RR of 0.90 (95% CI 0.62 to 1.29) [29]. Similarly, the same WHI study showed that women who used MHT in the first 10 years postmenopause had no increased coronary risk, while those who started using it 20 years after menopause did have a 52% higher risk (95% CI 7 .0 to 117) [30]. That is why the concept of the MHT window of opportunity has been proposed. In recent menopausal women with a healthy cardiovascular system, MHT would be cardioprotective, especially in women with premature ovarian insufficiency. In contrast, in women older than 60 years, with damaged arteries and unstable atherosclerotic plaques, MHT would be harmful.
Although the WHI study caused a massive abandonment of MHT, many women still continued with MHT, allowing further studies to be carried out, which continue and suggest a cardioprotective effect of hormone therapy. Schierbeck et al. [31] followed 1,006 women with an average age of 50 for 11 years, half of them treated with MHT; cardiovascular risk in treated women was 0.48 (95% CI 0.26 to 0.87). A Danish observational study that followed 489,105 women who used MHT between 1994 and 2009 showed that the risk of coronary death was reduced by 54% and that of stroke by 39% in women who used the therapy for more than 10 years [32]. Interestingly, Lobo et al. [33] compared MHT with other drugs used for the primary prevention of coronary disease, noting that while aspirin and statins do not have a clear preventive effect in women, MHT significantly decreases coronary risk in young women (RR 0.68, 95% CI 0.48 to 0.9).
Osteoporosis
Osteoporosis is a silent and emerging epidemic characterized by low bone mass and the deterioration of the bone microarchitecture, with the consequent increased risk of fractures. The etiopathogenic mechanisms through which estrogen deficiency affects bone mass are multiple. Estrogens can not only modify the neurochemistry involved in osteoporosis, as we have previously analyzed, but they also act directly on the bone tissue. At menopause, estrogen deficiency affects the normal cycle of bone turnover by increasing osteoclastic resorption without a compensatory increase in osteoblastic activity, with the amount of bone resorbed being greater than the amount deposited (imbalanced bone turnover), leading to net bone loss. This imbalance is the consequence of an increase in the production of tumor necrosis factor (TNF-α) and IL-1. These cytokines stimulate stromal cells/preosteoblasts to release various cytokines that modulate bone turnover (IL-6, M-CSF, IL-11, granulocyte-macrophage colony-stimulating factor, transforming growth factor). The final cytokine in the osteoclastogenesis cascade is RANKL (receptor activator of nuclear factor B ligand) which is synthesized by osteoblasts and binds to its RANK receptor in preosteoclasts, thus increasing their maturation. RANKL has a natural antagonist, osteoprotegerin (OPG), which is a soluble receptor that is secreted by cells of the stromal/osteoblastic line and its production is stimulated by estrogens, which also decrease M-CSF and RANK, necessary factors for the stimulation of osteoclasts, which ultimately results in decreased bone resorption with the use of estrogens [34].
Estrogen deficiency has been considered the fundamental mechanism of osteoporosis in both women and men, but epidemiological evidence in humans and studies in rodents indicate that aging and the associated increase in reactive oxygen species (ROS) play a major role in the generation and survival of osteoclasts, osteoblasts and osteocytes. Furthermore, the antioxidant action of FoxO transcription factors is essential for skeletal homeostasis at any age. The loss of estrogen decreases the defense against oxidative stress in the bone, and this explains the increase in bone resorption associated with the acute loss of this hormone. Furthermore, ROS-activated FoxOs cause disturbances of the Wnt/β-catenin pathway, leading to decreased osteoblastogenesis and increased osteoclatogenesis [35].
MHT counteracts the bone metabolic effects caused by estrogen deficit that occurs during the climacteric. A meta-analysis combining the results of osteoporosis prevention and treatment studies, both with estrogen alone and combined with progestins, showed a positive effect on bone mass at different measurement sites. After one year of MHT use, bone density increased 5.4% in the lumbar spine, 3.0% in the forearm, and 2.5% in the femoral neck; after two years of treatment, bone density increases another 1.5% on average at all studied sites [36]. Therefore, it is not surprising that MHT decreases the risk of fracture. A Cochrane database meta-analysis including 23 randomized placebo-controlled studies, including the WHI and HERS, and including 42,830 postmenopausal women showed that after 5.6 years of combined MHT, the absolute risk of fracture decreased by 86 cases per 1,000 treated women (compared to controls) and with estrogen alone the absolute risk after 7.1 years fell to 102 per 1,000 [37]. Probably, the decrease in the risk of fracture with MHT is not only due to its antiresorptive effect, but other positive effects of this therapy may also have an influence. The WHI study evaluated the effect of MHT on falls, a strong predictor of fracture, noting that women who take at least 80% of their therapy suffer fewer falls than control women [38]. The role that estrogens have in neuromuscular function may be influencing this, which is reflected in the existence of more than 200 genes that are modulated by estradiol and that have to do with muscle trophism. To this we must add that MHT decreases microRNAs (miRNA-182, miRNA-223), which activate anabolic pathways (IGF-1/PI3K/AKT) that positively impact the muscular system [39]. Estrogen deficiency may explain to some degree the presence of sarcopenia in elder women.
Cancer
Menopause per se does not imply an increased risk of cancer; however, changes in body composition that lead to increased fat, especially abdominal fat, may increase the risk of some cancers. Leptin is not only linked to caloric intake, but also to cell proliferation. Cancer cell growth is regulated by several cellular signals (STAT3, AP1, MAPK, ERKs) modulated by leptin and IL-6, which increase aromatase, estrogen synthesis, and alpha receptor activation in malignant cells, thus leading these cells to further proliferate [40]. Postmenopausal women with a body mass index (BMI) of 25, 30, and 35 kg/m2 yielded RRs of breast cancer of 1.02 (95% CI: 0.98-1.06), 1.12 (95% CI: 1.01-1.24), and 1.26 (95% CI: 1.07-1.50), respectively, when compared to women with a normal BMI. However, no significant result was observed in premenopausal women [41]. Obesity also increases the risk of endometrial, kidney, colon, and gallbladder cancer in postmenopausal women. A European study found that excess weight is responsible for 6% of all cancers in the European Union, varying between 3.9% in Denmark and 8.8% in Spain. The greatest impact of obesity in women was observed in endometrial cancer (obesity is responsible for 39% of cases), kidney (25%), and gallbladder (24%) [42]. This study estimates that 36,000 cases could have been avoided annually if obesity cases had been reduced by 50%.
The WHI estrogen-only study, after studying 10,000 hysterectomized women aged 50 to 79 followed for 10 years, showed a 23% lower risk of breast cancer in MHT users (HR 0.77; CI 95 % 0.62 to 0.95) [43]. In contrast, in women with combination therapy the risk of breast cancer increased by 28% (HR 1.28; 95% CI 1.11 to 1.48) [30]. However, the results of this last study have been challenged because the risk was calculated using the Cox proportional hazard model, without meeting the requirements of this model, which would invalidate the results. Applying another mathematical model (Royston & Palmer) to the same WHI patients, it was observed that, after five years, MHT users developed breast cancer 1 day earlier than controls [44]. Therefore, the described relationship between breast cancer and MHT remains quite unclear.
Colorectal cancer is the third leading cause of cancer death in US women. Observational studies suggest a reduced incidence of colorectal cancer and lower mortality in users of estrogen therapy, with or without associated progestins [45]. In the WHI trial, the use of conjugated equine estrogens (CEE) plus medroxyprogesterone acetate (MPA), but not estrogen alone, was associated with a reduced risk of colorectal cancer compared with placebo (HR, 0.62; 95% CI, 0.43-0.89) [46]. Despite the positive effect of combined MHT on colon cancer, it was described that diagnosed cancers were at a more advanced stage. The lower risk of colorectal cancer in combined MHT users was not observed during the post-intervention phase of the WHI at 13 years, and there was no difference in mortality from colorectal cancer [47]. The reasons related to the dissimilar results seen between observational studies and the WHI are not known.
Dementias
The 2015 World Alzheimer Report estimates that the prevalence of dementia in those over 60 years of age fluctuates between 4.6% in Central Europe and 8.7% in North Africa and the Middle East [48]. Epidemiology shows that Alzheimer's disease is more prevalent in women than in men. Likewise, experimental studies point out to the fact that in transgenic mice that overexpress characteristics of Alzheimer's disease, β-amyloid deposits are greater in females than in males. A higher prevalence of Alzheimer's disease has also been observed in postmenopausal women compared to men of the same age. Ovarian steroid deficiency deprives the female brain of a series of anti-Alzheimer effects that these hormones have, among which it is worth mentioning an improvement in synaptic connectivity, neurotransmission, less neuronal death and less accumulation of β-amyloid [49]. However, these positive estrogenic effects were not demonstrated in the WHI (WHIMS) study; the use of combined MHT in women older than 65 years doubled the risk of dementia [50]. Conversely, in women younger than 65 years, MHT is associated with a 65% decrease in the risk of Alzheimer's disease (OR 0.35; 95% CI: 0.19 to 0.66) [51]. These observations have led to the suggestion that there would be a 'window of opportunity' to prevent dementia with MHT; after the age of 60, hormone therapy could even increase the risk [52].
Estrogens facilitate higher cognitive functions by exerting effects on both the prefrontal cortex and the hippocampus, favoring synaptogenesis [53]. There are α, β, and transmembrane nuclear estrogen receptors (GPER) in the brain, the most important being α. In the absence of estrogen, the estrogen receptor α disappears from the brain, and can be re-induced only if estrogen is replaced relatively early. Women who are oophorectomized prior to menopause consistently show an increased risk of cognitive decline and dementia. Several studies have shown neuroprotection when MHT is started in the early postmenopause (mostly between the ages of 50 and 60) [54]. If hormone replacement is started later, on the other hand, the aforementioned cognitive benefits are not achieved. This phenomenon supports the “window of opportunity” theory for the brain benefits of MHT in postmenopause and helps understand the negative results of the WHIMS study.
Mortality
The positive effects of MHT on quality of life and the risk of chronic conditions should translate into lower mortality in women who use MHT. A meta-analysis of 19 randomized studies with 16,000 women followed for 83,000 patient-years showed that mortality in MHT users decreased by 27% (RR 0.73; 95% CI 0.52 to 0.96); when 8 observational studies were added to the previous analysis, the lower risk of death remained unchanged (RR 0.72; 95% CI 0.62 to 0.82) [55]. A large Finnish study that followed almost half a million women who used MHT for varying periods between 1994 and 2009 found that the risk of dying from any cause was reduced by 38% in women who used the therapy for more than 10 years [32]. In contrast, another meta-analysis of 10 studies, including 38,908 postmenopausal women, did not show a decrease in mortality with MHT [56]; however, this analysis included 27,347 WHI women who had a mean age of 63.2 years at baseline, which is outside the cardiovascular protective window of opportunity for MHT, as we have discussed previously. Therefore, everything seems to indicate that in women with recent menopause the use of MHT could be associated with lower mortality. Conversely, not using MHT could imply an even greater risk of mortality. Sarrel et al. [57] estimated that about 50,000 hysterectomized US women with ages 50 to 59 died earlier than they should have due to failure to use hormone therapy.
Conclusions
Menopause affects women’s quality of life and increases the risk of chronic conditions. By reducing climacteric symptoms, MHT improves quality of life and due to its multiple metabolic actions, especially at the level of the CNS, it reduces the risk of chronic conditions. Not prescribing MHT to women with climacteric symptoms, who do not have a contraindication for its use, would be depriving them of the opportunity of reversing these symptoms, improving their quality of life, and probably have a better quality of elder age.
Declarations of interest:
None.
Funding acknowledgement:
The elaboration of this manuscript did not receive any specific grant from funding agencies in the public, commercial, or non-for-profit sectors.