Introduction
Menopause is the functional failure of the ovaries, which leads to several changes in the functioning of the woman's body and an increased risk of developing diseases. Among the factors that lead to negative health outcomes resulting from menopause, the reduction in bone mineral density and sarcopenia can result in serious changes in the aging process [1].
Aging in women causes hormonal changes, such as a decrease in circulating estrogen due to natural or surgical menopause. These hormonal changes make postmenopausal women more susceptible to more rapid changes in body composition, muscle wasting, and abdominal obesity. Associated with this, changing to a more sedentary lifestyle affects general energy expenditure and basal metabolic rate [2].
One of the consequences of this rapid change in body composition is sarcopenia. The prevalence of sarcopenia may vary according to the different definitions and standards set by a given consensus. Regardless of the guideline used, the beginning of a greater loss of muscle mass and strength is associated with menopause due to hormonal factors and mainly due to the decrease in estrogen, considered a protein synthesis hormone. This phase is still accompanied by several modifiable factors, such as sedentary behavior and changes in dietary habits, resulting in associated comorbidities [3].
The decline in muscle mass throughout life can reach 50%, and only between 75 and 80 years these losses can reach about 25%; however, losses in postmenopausal women are more pronounced [4]. Although sarcopenia is unique, there are several consensuses with different classifications. For the Foundation of the National Institutes of Health (FNIH) Sarcopenia Project based on population studies defined sarcopenia with the same tools of other consensuses, but with new criteria, such as the analysis of muscle quality by Dual Energy X-ray absorptiometry (DXA) using the appendicular skeletal mass index (ASM/BMI) as the first criterion for calculating sarcopenia. This approach suggests that these references are stronger and directly correlated with weakness and physical performance [5].
The higher prevalence of sarcopenia observed in postmenopausal women highlights the need for continuous monitoring until becoming elder. These figures complications should alert public health to develop policies that promote healthy aging [6].
Methods
Sample
The descriptive analysis was carried out through a sample obtained from the database of the Laboratory of Densitometry at Federal University of Technology, Paraná, Brazil. All individuals who completed in the past 12 months in mentioned laboratory a detailed medical history questionnaire was included. The total number of eligible participants was 138; of which 27 participants were excluded, between men and women who did not perform any of the tests or had incomplete data. Hence, analysis was performed on a sample of 111 postmenopausal women who were also participating an exercise program promoted by the City of Curitiba, Brazil and underwent body composition assessment with Dual Energy X-ray absorptiometry (DXA) in last 12 months. The sample was divided into two groups for statistical purposes: women who had age of menopause onset 50 or less (Group BA; n=60) and those who had menopause onset after 50 years of age (Group AA; n=51).
Measurements
Height (m) was obtained using a stadiometer (Wiso 210, Santa Catarina, Brazil) and weight (Kg) was recorded on a digital scale (Wiso W801, Santa Catarina, Brazil). Handgrip strength (HGS) test was performed using a dynamometer (Jamar Hand Hydraulic Dynamometer) with a resolution of 2 kg. Three trials for each hand were performed with the participants in a sitting position, and the highest value of the dominant hand was used as a measure of HGS (expressed as Kg) for the analyses. For physical performance we used the Short Physical Performance Battery (SPPB), that assesses balance, gait speed and lower limb strength. The reference value used for the classification of HGS of participants followed the criteria of the National Institutes of Health Foundation (FNIH) (< 16 Kg). DXA (Hologic Discovery, Mississauga, Canada) determined body composition, body mass index (BMI), total body mass, appendicular skeletal on mass (ASM) to assess pre-sarcopenia (first criteria).
Statistical Analysis
Statistical analysis of retrospective investigation was performed with SPSS 17.0 software (SPSS Inc., Chicago, United States). Data of the sample are presented as means, standard deviations and minimum/maximum values. The Shapiro Wilk test was used to determine the normality of data distribution. To verify the relationship between variables, Pearson’s correlation test was performed, and to quantify the influence of one variable with each other, linear regression analysis was performed. A p value of < 0.05 was considered statistically significant.
Results
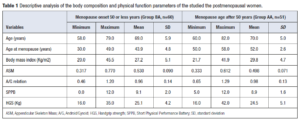
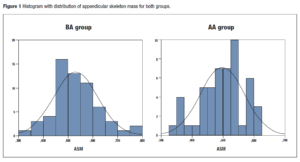
Descriptive analysis of the body composition and physical function parameters of the participating postmenopausal women are presented on Table 1. Women in AA group were older, had higher BMI and A/G relation values, and lower ASM, SPPB, and HGS values when compared with group BA. Histogram with distribution of ASM for both groups is presented in Figure 1.
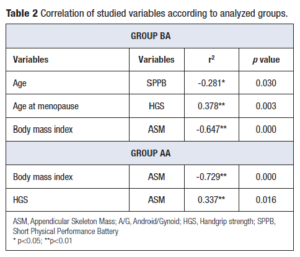
According to the FNIH, 56 women had pre-sarcopenia, which means that they had reference values below the recommended for only one criterion, which in this case represents low muscle mass as determined by DXA. Correlation of studied variables according to analyzed groups are presented in Table 2. In group BA, there was a significant inverse correlation between age and SPPB, and between BMI and ASM; with a positive correlation observed between age at menopause and HGS. In group AA, an inverse correlation was seen between BMI and ASM, with a positive correlation observed between HGS and ASM.
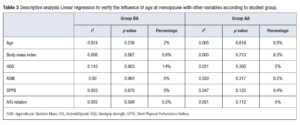
Linear regression to verify the influence of age at menopause with the analyzed variables according to the studied group is presented in Table 3. Less age at menopause (BA group) showed an important correlation of 14% with HGS values, while older age at menopause (Group AA) had a lower correlation with HGS values of only 2%.
Discussion
The decrease of estrogen levels, characteristic of postmenopausal women, appears to be related to a decline in muscle mass and muscle strength. However, the direct relationship of these effects is more complex and is linked to other interfering variables such as age itself and other hormonal influences [7].
For analysis regarding the relationship between age at onset of menopause and sarcopenia, we divided the sample according to age at menopause, those with lower age and those with higher age (Groups BA and AA, respectively). When comparing mean values between groups, in relation to body composition and physical function parameters, women in AA group presented worst results. Indeed, BMI and A/G relation values were higher; while ASM, SPPB and HGS values were lower when compared with BA group.
Menopause and aging are independently correlated with increased BMI. The influence on BMI, however, depends on the age of menopause, where women with late menopause have lower BMI compared to women with earlier age at menopause [8]. One study [9] analyzed the impact of age at natural menopause (ANM) on body composition and physical function in elderly women. The study included 1,582 women aged 70 to 84 years who were stratified into 4 groups according to their ANM: < 40 (premature natural menopause, PNM), 40 to 44 (early natural menopause, ENM), 45 to 54 (normal menopause, NM) and ≥55 (late menopause, LM) years. Although not statistically significant, differences related to physical function were found between the groups, in which the HGS in the PNM group was the lowest. There was also no statistically significant difference observed in body composition parameters measured by DXA, such as ASM index and trunk fat mass [9].
In this context, in the present study the correlation between the variables found different results for each group. In the BA group, age had a negative, yet weak, significant inverse correlation with SPPB; while age at menopause had a direct (moderate) significant correlation with HGS. ASM had an inverse (moderate) correlation with BMI. For the AA group, ASM presented a significant (strong) inverse correlation with BMI and a positive (moderate) correlation with HGS. In the same sense as our findings for the menopause group BA, one study of 756 postmenopausal Polish women (50 to 80 years) investigated the influence of age at onset of menopause on bone mineral density and muscle strength in groups of women who had menopause before and after 50 years of age. This research found a higher percentage of women with reduced bone mass and lower values of muscle strength in the group of women who had menopause earlier [1].
On the other hand, another study of 3,970 postmenopausal Korean women over the age of 40, which also used the FNIH sarcopenia criteria to classify sarcopenia, found that a longer reproductive period was significantly associated with a lower risk of developing sarcopenia. In addition, higher means of ASM/BMI measured by DXA were observed in the group with greater reproductive longevity [10].
Linear regression of our study demonstrated lower age at menopause (BA group) correlated to HGS with a 14%; while in Group AA, higher age at menopause onset had an influence on HGS of only 2% and A/G relation with 5%. For us, despite the shorter time of hormonal influence, the sample was generally represented by younger women compared to the AA group.
Our results were corroborated by a study that addressed the influence of age at menopause on bone and muscle mass that concluded that a higher percentage of women with reduced bone mass and lower level of general strength was found in the group of women who had early menopause. However, the rate of decline in HGS, upper and lower limb functional efficiency, and bone mineral density was greater in late menopausal women [11].
Similar results to ours were presented by a cross-sectional study that explored the prevalence of pre-sarcopenia in 340 middle-aged women. Women were divided into groups those with low muscle mass and those with normal muscle mass, and analyzed the correlation between HGS and abdominal obesity with low muscle mass. The results showed that a lower BMI value was associated with a greater chance of having low muscle mass (OR 7.1, 95% CI, 3.0-16.8, p value < 0.001), while higher values obtained in the HGS testing appeared to be a protective factor (OR 0.9, 95% CI, 0.8-1.0, p value = 0.04) [12].
As for the relationship between sarcopenia and changes in body composition, our study demonstrated that the inverse correlation between BMI and sarcopenia is maintained over the years after menopause, but this correlation becomes stronger, denoting lower values of muscle mass and greater adiposity (ASM -0.647** and -0.729**, for groups BA and AA, respectively). In the same line, one study concluded that combining sarcopenia with BMI seems to be a good measure of body composition to classify mortality risk among older adults, considering the authors that for the elderly, one should try to maintain a slightly higher BMI to try to avoid sarcopenia [13].
Regarding the onset of menopause and the effect on body composition, another investigation that included 1,393 women aged 47 to 55 years, divided into premenopausal, early perimenopausal, late perimenopausal and postmenopausal groups based on follicle-stimulating hormone concentration and bleeding diaries, concluded that the appendicular muscle mass and bone mineral density the femoral neck demonstrated a trend of significant linear decline in all analyzed groups, and the loss of muscle mass and bone density were associated with the transition to menopause [14].
Early diagnosis of pre-sarcopenia and maintenance of a healthy BMI can reduce the effects of protein catabolism and loss of muscle quality in middle-aged and older women [12]. For our sample, we found 56 participants with pre-sarcopenia and 55 with normal muscle mass. However, this classification was based on FNIH standard which uses muscle mass as a pre-sarcopenia classification criterion. In another research, that aimed at monitoring in 3 years the effects of muscle mass and performance in postmenopausal women (54.1 ± 4.3 years), there was an average loss of muscle mass of 0.6% per year, while for the muscle strength, losses reached 1.5% per year [15]. In addition, another study highlights the fact that the positive association of sarcopenia with falls and fractures in the elderly should increase the need for a more complete assessment of sarcopenia and thus define more objective interventions to minimize these outcomes that greatly reduce the quality of life of the older people [16].
Conclusion
In conclusion, the present research, based on women participating in a specific exercise program, demonstrated that earlier menopause had a significant influence on HGS while for the muscle quality, using our protocol to characterize pre-sarcopenia, there was no direct correlation with the onset of menopause in both groups. Thus, the use of muscle mass quality as the first classification criterion for sarcopenia (FNIH standard) does not seem to be the most appropriate for overall diagnosis; whereas, the use of HGS, as the first diagnostic criterion, may be more reliable for classifying sarcopenia.
Conflict of interest
The authors report having no conflicts of interest.
Funding
This study was funded by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior).