Introduction
Recurrent implantation failures are highly relevant issues in reproductive medicine. Despite the achievements of Assisted Reproductive Technologies (ART) in overcoming infertility, pregnancy rate remains low. According to the European IVF-monitoring Consortium, after IVF clinical pregnancy rate is about 35% [1]. It is recognized that an important reason for implantation failure is associated with insufficient endometrial receptivity [2]. There are different therapeutic options available to restore the endometrium in women with recurrent unsuccessful implantation, including photodynamic therapy, intrauterine infusion of autologous platelet-rich plasma and granulocyte-colony stimulating factor, scratching, manual physical therapy, though without sufficient scientific evidence for all.
New opportunities of tissue restoration are currently offered by modern device technologies using non-ablative erbium laser: Er:YAG laser with SMOOTH® mode (Fotona, Slovenia) [3]. Clinical and histological data related to the restoration effect of non-ablative laser therapy in tissues, such as skin [4], oral tissues [5], peripheral nerves [6], the vaginal mucosa [7,8], and urethral structure [9], are currently available. SMOOTH® mode of erbium laser has been successfully used for the treatment of women with vulvovaginal atrophy [10]. Vaginal application of 2940-nm of Er:YAG laser, using precisely controlled, sequentially packed pulse packets [11,12], distributes laser energy to achieve controlled heating of the mucous tissue, without ablation or overheating of the epithelium surface. Photothermal diffusion increases the local production of heat shock proteins and stimulates the expression of cytokines and growth factors such as transforming growth factor-β, basic fibroblast growth factor, epidermal growth factor (EGF), vascular endothelial growth factor (VEGF) among others, which cause an inflammatory response that activates fibroblasts for the production of new collagen and renewal of the extracellular matrix, thus improving vascularization and enhancing the oxygenation of the mucosal membranes [13,14]. A growing body of evidence suggests that non-ablative erbium laser is an effective and well-tolerated method for restoring vaginal function in women with vulvovaginal atrophy, pelvic organ prolapse and urinary incontinence. A positive effect on the appearance of the vaginal mucosa induced by laser thermal diffusion is rapid and prolonged, lasting up to 12 months after the treatment [15]. Relevant results are achieved one month after the first procedure (p<0.01) and continuously improved after two repeated procedures every four weeks [16,17]. The intraurethral laser application with a thin 4-mm-diameter canula provides additional therapeutic effect in patients with stress urinary incontinence [18,19].
According to the aforementioned information, we sought that laser non-ablative thermal energy can be delivered into the uterus and reach the basal layer of the endometrium, which consists of mycelium-like configuration of basalis glands, blood vessels, stem cells and fibroblasts, and thus stimulating epithelial and stromal cells to synthesize new functional glands and neoangiogenesis and achieve rejuvenation of the endometrium. The main objective of this study was to determine the effect and safety of intrauterine exposure with a non-ablative erbium laser on the endometrium. Another goal was to establish the efficacy of laser thermal diffusion in the preparation of the endometrium for frozen-thawed embryo transfer in order to improve the fertility rate. We present the first results of intrauterine irradiation of Er:YAG laser with SMOOTH® mode, describing histological, immunohistochemical (IHC) and sonographic changes in the endometrium, and clinical pregnancy rate after the laser treatment in infertile patients.
Methods
Study design and patients
This was an open prospective pilot study performed at ART-clinic “Vitalis” in Moscow, Russia from February 2022 to April 2023. The study was approved by the Ethics Committee of the F.I. Inozemtsev Academy of Medical Education, Saint Petersburg, Russia and was in accordance with the Helsinki Declaration.
Infertile women aged 24-45 years with a history of 1 to 5 implantation failures after fresh or frozen-thawed embryo transfer and planning the cycle for frozen-thawed high-quality embryo transfer, participated in the study after providing written informed consent. On the 6th - 10th day of the menstrual cycle they underwent a single procedure of intrauterine exposure of the endometrium and cervical mucosa with a non-ablative erbium laser using Fotona SP Dynamis laser system (Slovenia). Before and 1-2 months after laser treatment we evaluated the sonographic thickness of the endometrium in the middle of the menstrual cycle and a cervical PAP smear. Histological and IHC examinations of the endometrium on the 6th - 10th day of the menstrual cycle were performed before each laser procedure and in 33 women after 1-2 months of the treatment. Endometrial samples were obtained by office hysteroscopy or Pipelle biopsy.
A group of patients that underwent the frozen-thawed embryo transfer in the 1-4 months after the laser procedure was evaluated for implantation and clinical pregnancy rates. Exclusion criteria were cancer or infectious diseases of the pelvic organs, hyperplasia or malignancy of the endometrium, serious or chronic illnesses that could interfere with the study.
Laser procedure
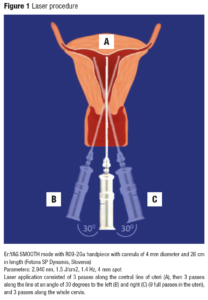
One laser session was performed for each patient using a non-ablative 2940-nm Er:YAG laser with a R09-2Gu handpiece and a cannula of 4 mm in diameter and 26 cm in length. The diameter of the laser spot size was 4 mm, with a pulse according to the SMOOTH® mode at a fluence of 1.5 J/cm2, and a frequency of 1.4 Hz. Parameters of the intrauterine laser procedure were derived from the intraurethral protocol (developed by Fotona) [3]. The procedures were performed in an outpatient clinical setting, without any preparation, anesthesia, or post-treatment medication.
Technique of the intrauterine laser application
First, we introduced the intrauterine probe through the cervix in order to measure the length of the cervix and the depth of the uterine cavity. Then we inserted a 4 mm-diameter laser cannula of the R09-2Gu handpiece into the uteri until it reached the bottom of the cavity (Figure 1). With the cannula fully inserted, we started delivering laser energy with 4 pulses on each location by pulling the canula 2.5 mm outwards until reaching the internal os of the cervix (approximate length 3 cm). Laser application consisted of 3 passes along the central line of the uteri, then 3 passes along the line at an angle of 30 degrees to the left and right (9 full passes in the uteri were completed). After that we performed 3 full passes along the whole cervical canal (approximate length 3 cm).
Assessment methods
Histological and IHC examinations of the endometrium were carried out in the laboratory INVITRO, Moscow. Sections of 3.5 μm thick were made from the endometrial tissue samples, fixed in 10% neutral buffered formalin solution for 24-48 hours, and mounted on highly adhesive slides, followed by routine histological examination with hematoxylin-eosin staining of the sample using light microscopy with a 4-40 x lens magnification. The IHC study was carried out according to the standard protocol using monoclonal antibodies in ready-made dilution to estrogen receptors α (ER-α) (Dako FLEX/Agilent, clone ER1), progesterone receptors (PR) (Dako FLEX/Agilent, clone PgR 636), CD138 (Dako FLEX/Agilent, clone MI15), VEGF (BioSystems Diagnostics, clone VG1), at 1:40 working dilution, and imaging systems EnVision™ FLEX+, High pH (Dako/Agilent), OptiView DAB IHC Detection Kit (Roche, Ventana). Expression was evaluated by light microscopy.
Expressions of CD138 and VEGF in the endometrium were assessed by a qualitative method, detecting their location: nuclear, membrane or cytoplasmic.
PR and ER expressions were evaluated by counting the number of stained cell nuclei of the endometrial glands and determining the ratio of stained/unstained cell nuclei per 100 counted cells in 10 representative fields of view at 40-fold magnification. The level of ER and PR expressions were assessed using the HISTO SCORE method, taking into account the number and intensity of stained cells of the epithelium of the glands and endometrial stroma and were calculated by the formula:
HS=1a+2b+3c, where a, b, c are % of weakly, moderately, and intensively stained cells, respectively, of the 1st, 2nd, and 3rd degree of expression expressed in points. At the same time: 0-10 points - no expression, 11-100 points - weak, 101-200 points - moderate, 201-300 points - high expression of ER and PR.
ThinPrepÒ technology with Papanicolaou staining was used for the cytological examination of the scraping of the cervix. The thickness of the endometrium in the middle of the menstrual cycle was assessed by ultrasound device HITACI ALOKA Prosaund alfa7.
Statistical analysis
Statistical analyses were performed using Microsoft Excel 2000 and Statistica 6.0 software. The normality of the distribution of variables was determined using the Kolmogorov–Smirnov test. Mean values with standard deviations (SD) were derived from normally distributed parameters. Normally distributed variables were compared using Student’s T test. Categorical variables were expressed as counts and percentages and compared using Fisher’s exact test. Standardized differences were calculated as means or proportion differences divided by the SD of the difference. A p value of < 0.05 was considered as statistically significant.
Results
Sixty-one patients underwent intrauterine laser procedures with the average duration of 13.1±2.7 minutes. During the laser irradiation of the uterine cavity and cervical canal, overall, patients did not have complaints or presented major discomfort, although some noted pain during the initial passage of the diagnostic uterine probe through the internal os of the cervical canal before the introduction of a cannula that delivers laser energy to the uterine cavity, which did not require anesthesia. There was no evidence of infectious complications after the intrauterine procedure, with spotting that lasted in a range of 1 to 3 days. Intrauterine and intracervical laser treatment was performed only after the exclusion of histological hyperplasia or malignancy in the endometrium and adverse changes in PAP smear.
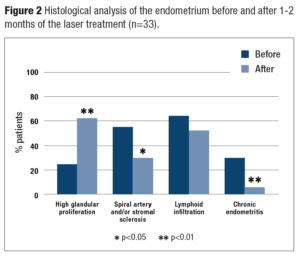
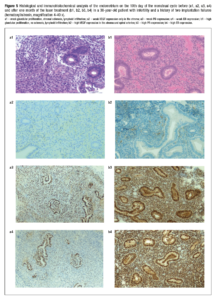
Comparative assessment of pre- and post-histological and IHC examinations of the endometrium of the 33 patients is demonstrated in Figure 2. Before the laser procedure there was a high glandular proliferation in the middle of the proliferative phase of the menstrual cycle in 24.2%, sclerosis of spiral arteries and/or stroma in 54.6%, and histological markers of chronic endometritis such as a positive expression of CD138 in combination with severe vascular and stromal sclerosis in 30.3%. Assessment of the endometrium on the same days of the cycle in 1-2 months after the laser treatment detected an increase in the number of the patients with high glandular proliferation to 60.6% (p=0.002), a decrease of sclerosis in the spiral arteries and/or stroma (30.3%; p=0.046), including those with chronic endometritis (6.1%; p=0.009), and significant reduction in its severity. Disseminated or focal lymphoid infiltration occurred in more than a half of the samples before treatment and although there was a decrease this was not significant.
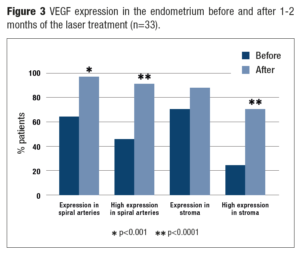
There was a significant improvement of VEGF expression in the endometrium, being a marker of vascular epithelium proliferation (Figure 3). Before the laser treatment VEGF expression was detected in the spiral arteries of 63.6% of the patients and 69.7% in the stroma, whereas after the laser procedure – in 97.1% (p=0.0007) and 87.9% (p=0.069), respectively, with most of specimens (90.9% and 69.7% vs initially 45.5% and 24.2%, respectively; p<0.0001) showing high expression.
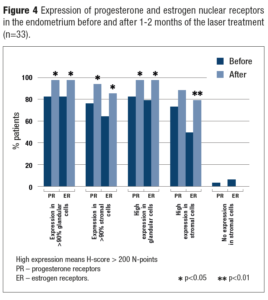
The same dynamics were observed with PR and ER expression (Figure 4). At baseline in more than 90% of glandular cells high expression (>200 N-points) of PR and ER was observed in 81.8% and 78.9% of the patients, and after the laser treatment this increased to 97.0% for both receptors (p=0.043). Expression of PR and ER in the >90% stromal cells were detected in only 75.8% and 63.6% of the patients before the treatment vs 93.9% (p=0.037) and 78.9% (p=0.009) after it. Moreover, there was a dramatic increase in the number of cells with high PR and ER expression from 63.6% and 48.5% to 84.9% and 78.9%, respectively (р=0.025 and р=0.009).
No histological destruction, necrosis, hyperplasia or malignancy in the endometrium, and no adverse changes in a PAP smears were detected before and after the laser treatment.
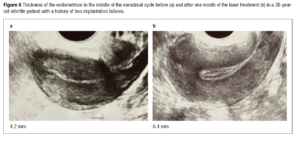
The average sonographic thickness of the endometrium in the middle of menstrual cycle was 5.9±0.87 mm before and 7.9±2.65 mm after the laser treatment (р<0.001).
Figure 5 presents the dynamics of histological and IHC data, and Figure 6, the sonographic thickness of the endometrium before and after one month of the laser procedure in a 38-year-old infertile woman. She became pregnant after frozen-thawed embryo transfer at 3 months after the laser treatment, and already had a live birth in April 2023.
Totally thirty-eight women (8 of them – twice) underwent the frozen-thawed embryo transfer into the uterine cavity at 1-4 months after the laser treatment. Rates of implantation and clinical pregnancy were 71.7% and 54.4%. Two of these women after the implantation failures conceived naturally. As a result, 27 of 38 infertility women (71.1%) achieved clinical pregnancy.
Discussion
The endometrium is a unique tissue that undergoes monthly cyclical changes resulting in menstruation, regeneration, proliferation, secretion and decidualization under the influence of ovarian steroids, which probably play a crucial role on implantation. Currently, physiotherapeutic methods of endometrial restoration are used to improve endometrial receptivity and fertility rate in women with recurrent implantation failure but without sufficient scientific evidence in this field. Thus, for example, localizing a photosensitizer into the uterine cavity followed by intrauterine exposure to low-intensity laser radiation with a power of 0.05 W/cm2 and an energy density of 40 J/cm2, considerably improved the histological structure and sonographic thickness of the endometrium in women with chronic endometritis [20,21]. Another study has described laser treatment of endometrial atrophy, in which a chlorophyll-containing drug is initially prescribed for 4-6 weeks, and then 3-6 sessions of low-intensity laser intrauterine therapy are performed [22]. These methods seem to ensure the restoration of the endometrium, though the absence of safety evidence of long-term exposure to the photosensitizing drugs in the uterus before the laser application, the lack of data of the depth and tissue modulation laser parameters, registry of pain during and after the procedure, appear to be the major disadvantages of photodynamic methods. Intrauterine administration of autologous platelet-rich plasma has also demonstrated some beneficial effects in the activation of growth factors, fibroblasts, neoangiogenesis and proliferation of the endometrium [23,24]. However, possible toxic effects of inflammatory factors in the body and biologically active components in the auto plasma should be considered. Intrauterine injection of granulocyte-colony stimulating factor for patients with thin endometrium who are undergoing IVF does not appear to be helpful [25]. In some ART clinics, endometrial scratching is used to overcome infertility. Mild damage to the endometrium increases local cytokines production involved in wound healing, which contributes to the natural mechanism of regeneration and preparation for “window of implantation” [26].
The present pilot study is the first to demonstrate the specifically targeted effect on the endometrium of Er:YAG laser with SMOOTH® mode induced by the Fotona SP Dynamis laser system and confirmed our hypothesis regarding its possible beneficial effect on women’s reproductive function.
Histological and IHC data 4-8 weeks after the laser procedure (time in which tissue remodeling occurs) clearly demonstrated the restoration effect on the structure and function of the endometrium. Proliferative activity of the endometrial glands and vascular epithelium in the first phase of menstrual cycle were improved, the endometrium receptor activity was restored in both the number of cells expressing nuclei ER and PR and the degree of their expression. The intrauterine laser procedure also showed a clear tendency of reduction in the prevalence of sclerosis in the endometrium, which is especially important for patients with chronic endometritis who had pronounced foci of sclerosis in the spiral arteries and stroma. Notably, non-ablative erbium laser irradiation did not cause fibrosis or hyperplasia in the endometrium. We also detected no destruction or ablation of the endometrium, despite the heating of the tissue during the procedure to 60-63°C. This thermal diffusion is provided by SMOOTH® mode 2940-nm Er:YAG laser with precisely controlled, sequentially packed pulse packets [11,12]. In contrast, the endometrium is fully destroyed under the same heating of other devices such as radiofrequency irradiation, neodymium YAG or argon laser, microwaves etc., which are used as alternatives to resectoscopic ablation or hysterectomy for the treatment of patients with heavy uterine bleeding [27-30].
Endometrial morphological and functional changes were evident after the laser procedure through the increase of its sonographically measured thickness (p < 0.05). The restorative effect of non-ablative laser energy on the endometrium seems to cause high clinical pregnancy rate (71.1%) in women who previously had infertility and had up to five unsuccessful implantations.
Additional advantage of our method is probable safety for the endometrium and the cervical mucosa which is based on strictly controlled and experimentally calculated parameters of thermal effects on the tissue developed for these types of laser systems and presented by our histological data and cervical PAP smears.
A limitation of the present pilot study is the relatively small number of patients, but our promising results indicate the need to continue further large and randomized studies in order to determine: the best number of laser procedures needed for the restoration of the endometrium, the influence of laser irradiation on the microbiome in the uteri and the cervix, the optimal period after treatment for the embryo transfer or natural conception, and also to evaluate the pregnancy outcomes after the intrauterine laser treatment.
In conclusion our pilot study of the intrauterine exposure with non-ablative erbium laser clearly demonstrated the improvement and synchronization with a phase of menstrual cycle of proliferative activity of the endometrium, reduction in the prevalence of sclerosis in the stroma and spiral arteries, restoration of PR and ER nuclei expression, and the increase in the sonographic thickness of the endometrium. Non-ablative thermal diffusion, generated by Er/YAG laser, seems to be safe for the endometrium and the cervix. The procedure is short in duration, does not require any anesthesia, or pre- or post-treatment medications. It seems that intrauterine laser exposure one to four months before the embryo transfer cycle can increase the pregnancy rate.