Introduction
Vaginal microbiota refers to the diverse microbial populations that biologically inhabit the female genital tract and plays a role in the homeostasis of the vaginal environment. The composition of the vaginal microbiota is highly variable among individuals and is influenced by factors including but not limited to diet, sexual activity, and hormonal changes. The composition of the vaginal microbiota can have an impact on women’s health throughout life, from adolescence and fertility to menopause and postmenopause.
The equilibrium of the vaginal microbiome is referred to as eubiosis. Eubiosis is defined as a condition of a healthy state, where all the elements of microorganisms of that specific organ work in synergy without causing disease. The most prevalent assumption is that to be in equilibrium, the vaginal microbiota must be dominated by a population of bacteria that produces lactic acid and hydrogen peroxide, which help maintain the acidic environment of the vagina and protect against the overgrowth of pathogenic bacteria, keeping the pH acid, and suppressing the activity of pathogens [1]. A relevant influence of the vaginal microbiome has been recently outlined on reproductive health and may be associated with various conditions such as bacterial vaginosis, recurrent vaginal infection and candidiasis [2].
In this narrative review, we describe the rationale for further research on vaginal microbiome analysis using an innovative method to target analysis of the microbiota composition and recommend personalized probiotics to improve the treatment for recurrent vulvovaginal infection (RVVI), including recurrent vul- vovaginal Candidiasis (RVVC), bacterial vaginosis (BV) and to support the management of the reproductive method.
. A literature search was conducted, and the methodology included a search of the PubMed, Google Scholar, and ResearchGate databases through 17 May 2023 for relevant studies using keywords and their combinations.
Recurrent vulvovaginal infection, candidiasis and bacterial vaginosis
RVVI is defined as four or more episodes of clinically apparent vulvovaginal infection per year [3]. A great example of those infections is RVVC, which is an infectious disease-related most frequently by the fungus Candida albicans, a highly common cause of vaginal infections in women of reproductive age, but also associated with C. glabrata, C. tropicalis, C. krusei and C. africana [4]. Symptoms of RVVC include vaginal itching, burning, and discharge [5]. Risk factors for RVVC include diabetes, pregnancy, use of antibiotics, and immunosuppression. The current therapeutical treatment of RVVC includes antifungal medications and lifestyle modifications [5].
BV is a common vaginal infection in women of reproductive age. It is caused by a shift in the normal balance of bacteria in the vagina, resulting in an overgrowth of certain bacteria. Symptoms may include thin, greyish-white vaginal discharge, an unpleasant fishy odor, and vaginal itching or burning. BV is generally not considered a sexually transmitted infection (STI), although it is more common in women who are sexually active [6]. International guidelines recommend antibiotic therapy, namely metronidazole, and clindamycin, per oral or vaginal route [7]. Antibiotics are effective as the cure rate ranges between 80-90%, but the recurrence of BV is widespread, such as up to 70% after one year. Newest data on the efficacy of alternative non-antibiotic options, namely probiotic products containing lactobacilli, lactic acid, and sucrose gel, alone or in combination with antibiotics are available showing promising results and even warrant more thorough trials with larger cohort sizes to provide sufficient evidence for these alternatives to be a part of official treatment recommendations [8-13].
Imbalances in the composition of the vaginal microbiome, such as reduced number of Lactobacillus species and an increase in the number of pathogenic bacteria, can lead to a higher risk of recurrent BV [14]. Restoring the vaginal microbiome through oral probiotic supplementation or intravaginal treatment with probiotic lactobacilli may help prevent recurrent BV [15].
Benefits of the supplementation with probiotics
Probiotics are defined as live microorganisms, predominantly bacteria, that improve health and overall well-being in humans, boosting the immune system, and reducing inflammation [16]. Evidence shows that oral probiotics have a promising scope in vaginal health by reducing vaginal pH and restoring normal vaginal flora. In addition, In addition, probiotic supplementation was found to be associated with higher rates of bacterial clearance, clinical and microbiological balance to decrease the symptoms of vaginal infections in women with the diagnosis of BV [17].
. Indicating probiotic supplementation in order to improve the vaginal microbiota eubiosis, reduced the recurrence of BV [18], and RVVC [19]. Several studies have shown a positive effect of probiotics [20]. However, they most often identify a population of responders and non-responders [21].
The vaginal microbiome was pioneering described by Professor Jack Ravel and includes four distinct community types: (1) the Lactobacillus crispatus-dominated community (L-dominant), (2) the Lactobacillus iners-dominant community (3) the bacterial vaginosis associated bacteria (BVAP) -dominant community (BVAB-dominant), and (4) the polymicrobial community (PM). These four community types have been studied extensively in the literature and have been associated with different health outcomes [22,23]. For example, BVAB-dominant and PM microbiomes are associated with an increased risk of STIs [24-26]. Additional understanding reveals that the vagina endures multifaceted changes from the neonatal to menopausal phases due to hormonal flux, metabolite deposition, and microbial colonization. These features have important implications in women’s health. Several pre-factors show dynamic characteristics according to the phases that shift the vaginal microbiota from anaerobes to aerobes which is a hallmark of a healthy vaginal environment. These factors include oestrogen levels, glycogen deposition, and vaginal microstructure. In the adult phase, Lactobacillus is highly dominant and regulates pH, adherence, aggregation, immune modulation, synthesis of bacteriocins, and biosurfactants (BSs) which are antagonistic to pathogens. Maternal factors are protective by favouring the colonization of lactobacilli in the vagina in the neonatal phase, which diminishes with age. The dominance of lactobacilli and dysbiosis in the adult phase depends on female intrinsic and extrinsic factors, which also vary between ethnicities [27,28].
The literature regarding the vaginal microbiome is increasingly highlighting the association between changes in the vaginal microbiota, RVVI and infertility, therefore their importance for women's health [29,30]. Literature is also increasingly showing that probiotic supplementation in responsive populations is an effective way to rebalance the vaginal microbiome [31]. The recent assumption of response to probiotics depending on the commu- nity state type (CST), and the development of personalized probiotics could significantly improve our management of RVVI and infertility, the aim of this paper is to evaluate validated assays to measure the CST in a precise manner as a tool to enable the personalized probiotic developments [32].
Discussion
Our hypothesis is that the response or non-response to probiotics can be due to the CST of the women taking probiotics [32]. We can therefore state, that knowing the a-priori CST would enable a better selection of probiotics and thus a higher response. The determination of CST requires precise, species-level discrimination of the most relevant micro-organisms in the vaginal microbiome, which is very difficult to obtain with 16S sequencing, a traditional method for the phenotypic identification of specific species, which in reality can be considered fragmentary because it only provides limited information on bacterial communities. More accurate identification is desirable and innovative analytical techniques for profiling and monitoring microbiome-strain interactions over time are emerging, such as next-generation sequencing (NGS) or Advanced Testing for Genetic Composition (ATGC) [32].
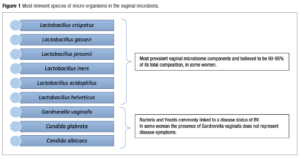
In recent years, the significant advancement in microbiome research has been strongly driven by methodological advances. Among these, molecular and high-throughput sequencing technologies, such as NGS have found emerging attention and have enabled the development of new methodologies that have an immediate effect on analysis results as their main goal. On the other side of the spectrum, we have seen advances in rtPCR to detect specific organisms with good reliability and specificity [33]. Recent developments in molecular techniques, combining targeted analysis of bacteria and fungus with precise quantification have produced ATGC. A targeted analysis looks only at a specific group of micro-organisms, the “target.” A targeted approach will always outperform an untargeted approach in a specific application [34]. It also offers the opportunity of including specific microbes as “targets.” These targets will be picked out of the total population with strain-level resolution. This will enable the diagnosis, and therefore treatment, of several sub-clinical infections which, although not dangerous, could cause In Vitro Fertilisation (IVF) failure and recurrent vaginal infections. Furthermore, in targeted analysis, the value lies in the target definition. Since Yoni has defined its target from knowledge built in-house, the value of the analysis lies with us. This makes our service unique. In the case of untargeted analysis, all the value lies in the analysis pipeline, and the same analysis can be performed by anyone [33]. Preliminary studies on the human microbiome with the application of targeted methods have certainly shown an increased richness of the microbiota [34,35]. For this reason, the implementation of innovative analytical techniques seems to be an appropriate method to monitor the changes in the vaginal composition of bacteria and its relationship with susceptibility to infection, the possibility of premature birth, and infertility [36]. These can be seen in Figure 1.
For Human Papiloma Virus (HPV), studies have suggested that a decrease in the number of Lactobacillus species is linked to hr-HPV infection. Infection severity is linked to Lactobacillus abundance and to cervical intraepithelial neoplasia (CIN) progression or regression [37]. The vaginal microbiome plays a critical role in determining the progression of female genital tract infections. Notably, the relative abundance of Lactobacillus is significantly high in normal microbiota, being represented predominantly by L. fermentum, L. gasseri, L. iners, L. jensenii, L. mucosae, L. ruminis, L. salivarius, L. coleohominis. In BV it was observed that Lactobacillus sp. decreased significantly. Recently, new evidence on Lactobacillus crispatus, a major determinant of vaginal health, was identified as heritable among European American women (narrow-sense heritability = 34.7%, P-value = 0.018). The heritability of L. crispatus is consistent with the reduced prevalence of adverse reproductive disorders among women of European ancestry, including BV and preterm birth [38-41].
The acid-producing abilities of Lactobacillus species have become the major criterion when evaluating their probiotic benefits [42,43]. Lactobacillus crispatus is thought to be a strong producer of lactic acid and thus is the basis of several probiotics [44-46]. L. crispatus-dominated vaginal microbiota are considered more beneficial than L. iners–dominated vaginal microbiota, because it is less likely to shift to dysbiosis and is associated with a lower prevalence of STIs [47].
The role of Lactobacillus iners is not clear in all populations but it is also commonly associated with vaginal infections and poor IVF outcomes. In some populations, L. iners is unable to sustain intravaginal acidity, which can lead to BV. Gardnerella vaginalis and L. iners are commonly found together without other Lactobacillus species [48].
Lactobacillus gasseri is very prevalent in the general population. It was identified in seminal fluids, follicular fluids, and embryo culture media of a group with unexplained infertility [49,50]. It has been demonstrated that L.gasseri significantly decreases sperm motility and that, as a whole, the seminal microbiome used for IVF impacts embryo quality and pregnancy rates. L. gasseri 33323 BS demonstrated anti-adhesion activity against the sexually transmitted pathogen Neisseria gonorrhoeae through the blocking of fibronectin, an extracellular matrix component on the epithelial cells [50].
Lactobacillus Jensenii is thought to be dominant in 5,3% women[21] while Lactobacillus in general are dominant in 73% of women’s microbiome, which can potentially contribute to lower vaginal pH (<4.5) through lactic acid production, which is believed to be a “protective environment” regarding vulvovaginal infections, pregnancy, and fertility outcomes as well [51].
Lactobacillus acidophilus is an important vaginal microbiome component due to its relation with acid lactic production and protective properties. The strain KS400 has been proven to produce bacteriocin through fermentation and inhibited the growth of urogenital pathogens such as G. vaginalis, S. agalactiae, and P. aeruginosa[42]. Among the most prevalent fungi in the vaginal microbiota, the genera Candida is the most recurrent and Candida albicans is the most prevalent in women’s vaginal microbiome. It is present in an average of 30% of the female population. The current literature scores C. albicans as significantly absent in women with microbiota considered healthy and present in women with vulvovaginal candidiasis (VVC). Its prevalence in women with the diagnosis of VVC can vary greatly according to the region in which the study has taken place [52].
Candida glabrata is a prevalent non-albicans specie among the Candida spp. It is believed to be related to up to 17% of VVC. It has been frequently related to RVVI because of its resistance to the Azole class of medications. C. glabrata is 10 times less sensitive to miconazole than is C. albicans (the most frequent one linked to VVC). For this reason, C. glabrata is linked to RVVC [53]. A lot of women were also reported to have Candida tropicalis, another Candida non-albicans species. As noticed previously, non-albicans candida species create a major problem in the treatment of RVVI. The incidence of non-albicans species increases, and they demonstrate elevated resistance to antifungal agents [54].
Conclusion
The literature regarding the vaginal microbiome is increasingly highlighting its importance for women's health. There is a clear connection between the composition of the vaginal microbiome and the risk of developing vaginal infections, reproductive health issues, and other medical conditions. Probiotics in a responsive female population are an effective way to rebalance the vaginal microbiome and achieve eubiosis. If we assume that the response to probiotics depends on the CST, then the development of personalized probiotics could significantly improve our management of RVVI, reproductive health issues, and other medical conditions. Validated assays to measure the CST in a precise manner will enable these developments. Further research is also needed to explore the full potential of personalized probiotics and their impact on women’s health.